Application of Quality by Design Approach in the Optimization and Development of the UPLC Analytical Method for Determination of Fusidic Acid in Pharmaceutical Products
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Methods
2.2.1. Design of Experiment (DoE)
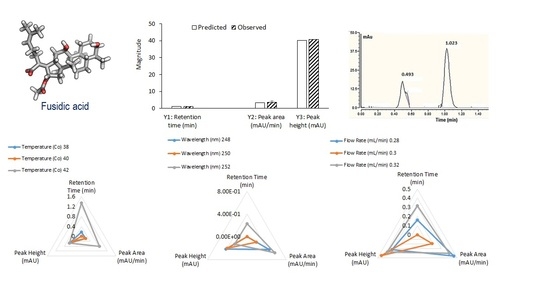
2.2.2. System and Conditions
2.2.3. Preparation of Standard Stock Solution, Calibration, and Quality Control Samples
2.2.4. UPLC Analytical Validation
Linearity and Range
Accuracy and Precision
Robustness
System Suitability
Forced Degradation Studies
2.2.5. Application of the Developed Method on Content Uniformity
3. Results and Discussion
3.1. Design of Experiment (DoE)
3.1.1. Effect on Retention Time (RT)
3.1.2. Effect on Peak Area
3.1.3. Effect on Peak Height
3.2. Optimization of UPLC Conditions for FU Analysis
3.3. Method Validation
3.3.1. Linearity
3.3.2. Limit of Detection and Limit of Quantitation
3.3.3. Accuracy and Precision
3.3.4. Robustness
3.3.5. System Suitability
3.4. Forced Degradation Study
3.5. Analysis of Drug Content Uniformity in Marketed Cream
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ueda, C.T.; Shah, V.P.; Derdzinski, K.; Ewing, G.; Flynn, G.; Maibach, H.; Marques, M.; Rytting, H.; Shaw, S.; Thakker, K.; et al. Topical and Transdermal Drug Products. Dissolut. Technol. 2010, 17, 12–25. [Google Scholar] [CrossRef]
- The United States Pharmacopeial Convention. Topical and Transdermal Drug Products. In Proceedings of the Pharmacopeial Forum, Brussels, Belgium, 25 March 2009; Volume 35, pp. 750–764. [Google Scholar]
- Marsona, B.M.; Concentino, V.; Junkert, A.M.; Fachi, M.M.; Vilhena, R.O.; Pontarolo, R. Validation of analytical methods in a pharmaceutical quality system: An overview focused on HPLC methods. Química Nova 2020, 43, 1190–1203. [Google Scholar] [CrossRef]
- Rina, R.; Baile, M.; Jain, A. A Review: Analytical Method Development and Validation. Syst. Rev. Pharm. 2021, 12, 450–454. [Google Scholar]
- FDA. Analytical Procedures and Methods Validation: Chemistry, Manufacturing, and Controls; Federal Register: Washington, DC, USA, 2000; Volume 65, pp. 52776–52777. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/analytical-procedures-and-methods-validation-drugs-and-biologics (accessed on 1 May 2023).
- Long, J.; Ji, W.; Zhang, D.; Zhu, Y.; Bi, Y. Bioactivities and Structure–Activity Relationships of Fusidic Acid Derivatives: A Review. Front. Pharmacol. 2021, 12, 759220. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.; Turnidge, J. Fusidic acid in vitro activity. Int. J. Antimicrob. Agents 1999, 12, S45–S58. [Google Scholar] [CrossRef] [PubMed]
- Tansirichaiya, S.; Goodman, R.N.; Guo, X.; Bulgasim, I.; Samuelsen, Ǿ.; Al-Haroni, M.; Roberts, A.P. Intracellular Transposition and Capture of Mobile Genetic Elements following Intercellular Conjugation of Multidrug Resistance Conjugative Plasmids from Clinical Enterobacteriaceae Isolates. Microbiol. Spectr. 2022, 10, e02140-21. [Google Scholar] [CrossRef] [PubMed]
- Wieland, M.; Holm, M.; Rundlet, E.J.; Morici, M.; Koller, T.O.; Maviza, T.P.; Pogorevc, D.; Osterman, I.A.; Müller, R.; Blanchard, S.C.; et al. The cyclic octapeptide antibiotic argyrin B inhibits translation by trapping EF-G on the ribosome during translocation. Proc. Natl. Acad. Sci. USA 2022, 119, e2114214119. [Google Scholar] [CrossRef] [PubMed]
- Cé, R.; Pacheco, B.Z.; Ciocheta, T.M.; Barbosa, F.S.; Alves, A.; Dallemole, D.R.; Lavayen, V.; Guterres, S.S.; Steppe, M.; Pohlmann, A.R. Antibacterial activity against Gram-positive bacteria using fusidic acid-loaded lipid-core nanocapsules. React. Funct. Polym. 2021, 162, 104876. [Google Scholar] [CrossRef]
- Bourles, A.; Tristan, A.; Vandenesch, F.; Bes, M.; Laurent, F.; Ranc, A.G.; Kainiu, M.; Gourinat, A.C.; Biron, A.; Cazarola, C.; et al. A fusidic acid-resistant (PVL+) clone is associated with the increase in methicillin-resistant Staphylococcus aureus in New Caledonia. J. Glob. Antimicrob. Resist. 2020, 30, 363–369. [Google Scholar] [CrossRef]
- Shewale, S.; Undale, V.; Deshmukh, A.; Wawale, V.; Gundecha, S.; Bhalchim, V. Assessment of Fusidic acid (FA) and Beclomethasone Dipropionate (BD) In Semi-solid Dosage Form Using Validated High-Performance Liquid Chromatography Method. J. Pharm. Negat. 2022, 13, 1272–1285. [Google Scholar]
- Lee, G.; Choi, M.; Truong, Q.-K.; Mai, X.-L.; Kang, J.-S.; Woo, M.-H.; Na, D.-H.; Chun, I.-K.; Kim, K.H. Development of HPLC assay method of fusidate sodium tablets. Anal. Sci. Technol. 2017, 30, 154–158. [Google Scholar]
- Jakasaniya, M.A.; Shah, J.S.; Maheswari, D.G. Simultaneous estimation of clobetasol propionate and fusidic acid in cream dosage form by reversed phase high performance liquid chromatographic method. Pharmacophore 2014, 5, 231–238. [Google Scholar]
- Nawaz, M.; Arayne, M.; Sultana, N.; Haider, A.; Hisaindee, S. Simultaneous determination of fusidic acid and steroids from bulk drugs and human plasma by reversed phase HPLC. Acta Chromatogr. 2014, 6, 57–66. [Google Scholar] [CrossRef]
- Peter, J.D.; Jehl, F.; Pottecher, T.; Dupeyron, J.P.; Monteil, H. Pharmacokinetics of Intravenous Fusidic Acid in Patients with Cholestasis. Antimicrob. Agents Chemother. 1993, 37, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, P.; Tsaganos, T.; Plachouras, D.; Carrer, D.P.; Papadopoulos, A.; Giamarellou, H.; Kanellakopoulou, K. In vitro elution of moxifloxacin and fusidic acid by a synthetic crystallic semihydrate form of calcium sulphate (Stimulan). Int. J. Antimicrob. Agents 2008, 32, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, Y. Quality control analytical methods—Switch from HPLC to UPLC. In Proceedings of the 4th International Summit on GMP, GCP & Quality Control, Hyderabad, India, 26–28 October 2015. [Google Scholar]
- Dolan, J. A Guide to HPLC and LC-MS Buffer Selection. Available online: http://www.ace-hplc.com/ (accessed on 24 April 2023).
- Sharmaa, G.; Thakura, K.; Razab, K.; Katarea, O.P. Stability kinetics of fusidic acid: Development and validation of stability indicating analytical method by employing Analytical Quality by Design approach in medicinal product(s). J. Chromatogr. B 2019, 1120, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Waters Corporation; Milford, M.A. Effect of Temperature on Column Pressure, Peak Retention Time and Peak Shape. Available online: Chromeextension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.waters.com/webassets/cms/library/docs/watersamd30.pdf (accessed on 22 June 2022).
- Waterlot, C.; Goulas, A. Temperature Effects on Retention and Separation of PAHs in Reversed-Phase Liquid Chromatography Using Columns Packed with Fully Porous and Core-Shell Particles. J. Chem. 2016, 2016, 7294105. [Google Scholar] [CrossRef]
- Yang, Y.; Lamm, L.J.; He, P.; Kondo, T. Temperature effect on peak width and column efficiency in subcritical water chromatography. J. Chromatogr. Sci. 2002, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Malenović, A.; Ivanović, D.; Medenica, M.; Jančić, B. Application of the response surface methodology for RP-HPLC analysis of lidocaine and cetrimonium bromide. Acta Chim. Slov. 2004, 51, 559–566. [Google Scholar]
- Ibrahim, M.A.; Alshora, D.H.; Alowayid, M.A.; Alanazi, N.A.; Almutairi, R.A. Development and Validation of a Green UPLC Analytical Procedure for Glibenclamide Determination in Pharmaceutical Product Using Response Surface Methodology. Orient. J. Chem. 2022, 38, 865–874. [Google Scholar] [CrossRef]






| Independent Variables (Factors) | Low (−1) | Middle (0) | High (+1) |
|---|---|---|---|
| X1: Formic acid (%) | 0.1 | 0.55 | 1.0 |
| X2: Column temperature (°C) | 25.0 | 32.5 | 40.0 |
| Dependent Variable (Response): | |||
| Y1: Retention time (min) | |||
| Y2: Peak area (mAu/min) | |||
| Y3: Peak area (mAu) |
| Response | Source | Sum of Squares | p-Value |
|---|---|---|---|
| Retention Time | A-Formic acid concentration | 0.0080 | 0.1180 |
| B-Temperature | 0.0508 | 0.0120 | |
| AB | 0.0108 | 0.0858 | |
| A2 | 0.0044 | 0.2065 | |
| B2 | 0.0010 | 0.5003 | |
| Peak Area | A-Formic acid concentration | 0.0351 | 0.3056 |
| B-Temperature | 0.0641 | 0.1946 | |
| AB | 0.0756 | 0.1682 | |
| A2 | 0.0371 | 0.2944 | |
| B2 | 0.0304 | 0.3349 | |
| Peak Height | A-Formic acid concentration | 8.52 | 0.0041 |
| B-Temperature | 0.1900 | 0.3197 | |
| AB | 0.1368 | 0.3871 | |
| A2 | 0.2515 | 0.2645 | |
| B2 | 0.0989 | 0.4537 |
| Run | X1, Formic Acid (%) | X2, Temperature (°C) | Retention Time (min) | Peak Area (mAu/min) | Peak Height (mAu) |
|---|---|---|---|---|---|
| 1 | 0.1 | 25.0 | 1.128 ± 0.002 | 2.968 ± 0.030 | 41.177 ± 0.154 |
| 2 | 0.1 | 32.5 | 1.072 ± 0.005 | 3.241 ± 0.107 | 40.152 ± 1.379 |
| 3 | 0.1 | 40.0 | 1.021 ± 0.008 | 3.545 ± 0.059 | 40.227 ± 1.254 |
| 4 | 0.55 | 25.0 | 1.145 ± 0.004 | 3.01 ± 0.094 | 39.545 ± 0.320 |
| 5 | 0.55 | 32.5 | 1.081 ± 0.007 | 3.079 ± 0.043 | 39.862 ± 0.235 |
| 6 | 0.55 | 40.0 | 1.022 ± 0.008 | 3.026 ± 0.195 | 39.637 ± 0.702 |
| 7 | 1.0 | 25.0 | 1.136 ± 0.002 | 2.977 ± 0.059 | 38.370 ± 0.197 |
| 8 | 1.0 | 32.5 | 1.046 ± 0.017 | 3.314 ± 0.552 | 37.877 ± 0.576 |
| 9 | 1.0 | 40.0 | 0.862 ± 0.042 | 3.004 ± 0.002 | 38.643 ± 0.420 |
| Optimized Independent Parameters | Response | |||
|---|---|---|---|---|
| Type | Desirability | Predicted | Observed | |
| Formic acid (X1) = 0.1% | Y1: Retention time (min) | Minimum | 1.03 | 1.02 ± 0.019 |
| Y2: Peak area (mAU/min) | Maximum | 3.45 | 3.70 ± 0.0.043 | |
| Temperature (X2) = 40 °C | Y3: Peak height (mAU) | Maximum | 40.23 | 40.64 ± 0.77 |
| Nominal Concentration (ppm) | 1 | 50 | 75 | |
|---|---|---|---|---|
| Inter-Day | Day-1 | 0.885 ± 0.013 | 49.932 ± 0.015 | 75.058 ± 1.097 |
| 1.556 | 0.031 | 1.462 | ||
| Day-2 | 0.880 ± 0.005 | 49.707 ± 0.326 | 80.491 ± 1.566 | |
| 0.592 | 0.650 | 1.940 | ||
| Day-3 | 0.879 ± 0.013 | 55.168 ± 0.584 | 81.176 ± 0.117 | |
| 1.488 | 1.060 | 0.144 | ||
| Intra-day (on day 1) | ||||
| % Recovery | 89.353 ± 1.564 | 100.060 ± 1.41 | 100.922 ± 1.463 | |
| RSD% | 1.556 | 0.031 | 1.462 | |
| Wavelength (nm) | |||
|---|---|---|---|
| 248 | 250 | 252 | |
| Retention Time (min) | 1.37347 × 10−14 | 1.37347 × 10−14 | 0.232 |
| Peak Area (mAU/min) | 0.452 | 0.186 | 0.567 |
| Peak Height (mAU) | 0.433 | 0.399 | 0.247 |
| Temperature (°C) | |||
| 38 | 40 | 42 | |
| Retention Time (min) | 0.171 | 1.37347 × 10−14 | 1.334 |
| Peak Area (mAU/min) | 0.187 | 0.186 | 0.831 |
| Peak Height (mAU) | 0.565 | 0.399 | 0.551 |
| Flow Rate (mL/min) | |||
| 0.28 | 0.3 | 0.32 | |
| Retention Time (min) | 0.163 | 1.37347 × 10−14 | 0.319 |
| Peak Area (mAU/min) | 0.459 | 0.186 | 0.399 |
| Peak Height (mAU) | 0.319 | 0.454 | 0.374 |
| Tailing Factor (T) | Asymmetry Factor (AS) | Area Reproducibility (%RSD) | |
|---|---|---|---|
| Fusidic acid | 1.22 | 1.375 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.; Alhabib, N.A.; Alshora, D.; Bekhit, M.M.S.; Taha, E.; Mahdi, W.A.; Harthi, A.M. Application of Quality by Design Approach in the Optimization and Development of the UPLC Analytical Method for Determination of Fusidic Acid in Pharmaceutical Products. Separations 2023, 10, 318. https://doi.org/10.3390/separations10050318
Ibrahim M, Alhabib NA, Alshora D, Bekhit MMS, Taha E, Mahdi WA, Harthi AM. Application of Quality by Design Approach in the Optimization and Development of the UPLC Analytical Method for Determination of Fusidic Acid in Pharmaceutical Products. Separations. 2023; 10(5):318. https://doi.org/10.3390/separations10050318
Chicago/Turabian StyleIbrahim, Mohamed, Nasser Ali Alhabib, Doaa Alshora, Mounir M. Salem Bekhit, Ehab Taha, Wael A. Mahdi, and Abdulelah M. Harthi. 2023. "Application of Quality by Design Approach in the Optimization and Development of the UPLC Analytical Method for Determination of Fusidic Acid in Pharmaceutical Products" Separations 10, no. 5: 318. https://doi.org/10.3390/separations10050318
APA StyleIbrahim, M., Alhabib, N. A., Alshora, D., Bekhit, M. M. S., Taha, E., Mahdi, W. A., & Harthi, A. M. (2023). Application of Quality by Design Approach in the Optimization and Development of the UPLC Analytical Method for Determination of Fusidic Acid in Pharmaceutical Products. Separations, 10(5), 318. https://doi.org/10.3390/separations10050318