Three-Dimensional Ternary rGO/VS2/WS2 Composite Hydrogel for Supercapacitor Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of Graphene Oxide and Preparation of Vanadium Disulfide (VS2)
2.3. Development of rGO Hydrogels
2.4. Binary Composite Hydrogel (rGO-VS2 (rG-V)) Preparation
2.5. Preparation of rG-V-W Hydrogel
2.6. Structural/Microstructural Characterization and Electrochemical Measurements
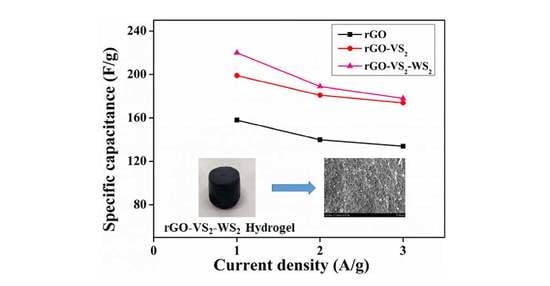
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, J.; Luo, Q.; Hou, Q.; Chen, H.; Liu, T.; He, H.; Wang, J.; Shao, Q.; Dong, M.; Wu, S.; et al. Suppressing Charge Recombination and Ultraviolet Light Degradation of Perovskite Solar Cells Using Silicon Oxide Passivation. ChemElectroChem 2019, 6, 3167–3174. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, X.; Lu, J.; Geng, X.; Wan, Y.; Wu, M.; Yang, P. Substrate effects on the CVD growth of MoS2 and WS2. J. Mater. Sci. 2019, 55, 990–996. [Google Scholar] [CrossRef]
- Liu, M.; Meng, Q.; Yang, Z.; Zhao, X.; Liu, T. Ultra-long-term cycling stability of an integrated carbon–sulfur membrane with dual shuttle-inhibiting layers of graphene “nets” and a porous carbon skin. Chem. Commun. 2018, 54, 5090–5093. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qin, J.; Hu, Q.; Wang, D.; Wang, F.; Zhong, Y.; Zhang, J.; Zhou, H.; Dong, M.; Hu, C. Theoretical investigation of molybdenum/tungsten-vanadium solid solution alloy membranes: Thermodynamic stability and hydrogen permeation. J. Membr. Sci. 2020, 608, 118200. [Google Scholar] [CrossRef]
- Wei, H.G.; Wang, H.; Li, A.; Cui, D.P.; Zhao, Z.N.; Chu, L.Q.; Wei, X.; Wang, L.; Pan, D.; Fan, J.C.; et al. Multifunctions of Polymer Nanocomposites: Environmental Remediation, Electromagnetic Interference Shielding, And Sensing Applications. ChemNanoMat 2020, 6, 174–184. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, H.; Su, W.; Wang, T.; Wang, X.; Chen, T.; Huo, T.; Dang, F.; Dong, M.; Wang, C.; et al. Trace bismuth and iodine co-doping enhanced thermoelectric performance of PbTe alloys. J. Phys. D Appl. Phys. 2020, 53, 245501. [Google Scholar] [CrossRef]
- Tan, J.; Li, D.; Liu, Y.; Zhang, P.; Qu, Z.; Yan, Y.; Hu, H.; Cheng, H.; Zhang, J.; Dong, M. A self-supported 3D aerogel network lithium–sulfur battery cathode: Sulfur spheres wrapped with phosphorus doped graphene and bridged with carbon nanofibers. J. Mater. Chem. A 2020, 8, 7980–7990. [Google Scholar] [CrossRef]
- Li, X.; Zhao, W.; Yin, R.; Huang, L.; Qian, X. A highly porous polyaniline-graphene composite used for electrochemical supercapacitors. Eng. Sci. 2018, 3, 89–95. [Google Scholar] [CrossRef]
- Dong, H.; Li, Y.; Chai, H.; Cao, Y.; Chen, X. Hydrothermal Synthesis of CuCo S Nano-structure and N-Doped Graphene for High- 2 4 Performance Aqueous Asymmetric Supercapacitors. ES Energy Environ. 2019, 4, 19–26. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, M.; Yin, X.; Shao, Q.; Lu, N.; Feng, Y.; Lu, Y.; Wujcik, E.K.; Mai, X.; Wang, C.; et al. Tuning polyaniline nanostructures via end group substitutions and their morphology dependent electrochemical performances. Polymer 2018, 156, 128–135. [Google Scholar] [CrossRef]
- Yang, L.; Guo, X.; Jin, Z.; Guo, W.; Duan, G.; Liu, X.; Li, Y. Emergence of melanin-inspired supercapacitors. Nano Today 2021, 37, 101075. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Duan, G.; Zhao, L.; Zhang, C.; Chen, L.; Zhang, Q.; Liu, K.; Wang, F. Pyrolysis of zinc salt-treated flax fiber: Hierarchically porous carbon electrode for supercapacitor. Diam. Relat. Mater. 2022, 129, 109339. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Chen, Y.; Cheng, K.; Yan, J.; Zhu, K.; Ye, K.; Wang, G.; Zhou, L.; Cao, D. Freestanding 3D Polypyrrole@ reduced graphene oxide hydrogels as binder-free electrode materials for flexible asymmetric supercapacitors. J. Colloid Interface Sci. 2019, 536, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, H.; Li, A.; Li, H.; Cui, D.; Dong, M.; Lin, J.; Fan, J.; Zhang, J.; Hou, H. Advanced porous hierarchical activated carbon derived from agricultural wastes toward high performance supercapacitors. J. Alloy. Compd. 2020, 820, 153111. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, J.; Xu, L.; Liu, H.; Zhou, B.; Tang, Y.; Zhu, L.; Jiang, X.; Jiang, X. Facile synthesis of a graphene/nickel-cobalt hydroxide ternary hydrogel for high-performance supercapacitors. J. Colloid Interface Sci. 2018, 531, 593–601. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Cao, L.; Li, H.; Xu, Z.; Zhang, H.; Ding, L.; Wang, S.; Zhang, G.; Hou, H.; Xu, W.; Yang, F.; et al. Comparison of the heteroatoms-doped biomass-derived carbon prepared by one-step nitrogen-containing activator for high performance supercapacitor. Diam. Relat. Mater. 2021, 114, 108316. [Google Scholar] [CrossRef]
- Wang, F.; Cheong, J.Y.; He, Q.; Duan, G.; He, S.; Zhang, L.; Zhao, Y.; Kim, I.-D.; Jiang, S. Phosphorus-doped thick carbon electrode for high-energy density and long-life supercapacitors. Chem. Eng. J. 2021, 414, 128767. [Google Scholar] [CrossRef]
- Duan, G.; Zhao, L.; Chen, L.; Wang, F.; He, S.; Jiang, S.; Zhang, Q. ZnCl 2 regulated flax-based porous carbon fibers for supercapacitors with good cycling stability. New J. Chem. 2021, 45, 22602–22609. [Google Scholar] [CrossRef]
- Cao, L.; Li, H.; Liu, X.; Liu, S.; Zhang, L.; Xu, W.; Yang, H.; Hou, H.; He, S.; Zhao, Y. Nitrogen, sulfur co-doped hierarchical carbon encapsulated in graphene with “sphere-in-layer” interconnection for high-performance supercapacitor. J. Colloid Interface Sci. 2021, 599, 443–452. [Google Scholar] [CrossRef]
- Guo, W.; Guo, X.; Yang, L.; Wang, T.; Zhang, M.; Duan, G.; Liu, X.; Li, Y. Synthetic melanin facilitates MnO supercapacitors with high specific capacitance and wide operation potential window. Polymer 2021, 235, 124276. [Google Scholar] [CrossRef]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, C.; Tang, C.; Bi, S.; Wang, Q.; Du, D.; Song, J. High output nano-energy cell with piezoelectric nanogenerator and porous supercapacitor dual functions—A technique to provide sustaining power by harvesting intermittent mechanical energy from surroundings. Nano Energy 2016, 21, 209–216. [Google Scholar] [CrossRef]
- EMeyer, E.; Bede, A.; Mutukwa, D.; Taziwa, R.; Zingwe, N. Optimization, and analysis of carbon supported VS2 nanocomposites as potential electrodes in supercapacitors. J. Energy Storage 2019, 27, 101074. [Google Scholar]
- Murugan, A.V.; Quintin, M.; Delville, M.-H.; Campet, G.; Vijayamohanan, K. Entrapment of poly (3, 4-ethylenedioxythiophene) between VS 2 layers to form a new organic–inorganic intercalative nanocomposite. J. Mater. Chem. A 2005, 15, 902–909. [Google Scholar] [CrossRef]
- Huang, Z.; Han, X.; Cui, X.; He, C.; Zhang, J.; Wang, X.; Lin, Z.; Yang, Y. Vertically aligned VS 2 on graphene as a 3D heteroarchitectured anode material with capacitance-dominated lithium storage. J. Mater. Chem. A 2020, 8, 5882–5889. [Google Scholar] [CrossRef]
- Torad, N.L.; Hu, M.; Kamachi, Y.; Takai, K.; Imura, M.; Naito, M.; Yamauchi, Y. Facile synthesis of nanoporous carbons with controlled particle sizes by direct carbonization of monodispersed ZIF-8 crystals. Chem. Commun. 2013, 49, 2521–2523. [Google Scholar] [CrossRef]
- Pang, S.; Tsao, H.N.; Feng, X.; Müllen, K. Patterned graphene electrodes from solution-processed graphite oxide films for organic field-effect transistors. Adv. Mater. 2009, 21, 3488–3491. [Google Scholar] [CrossRef]
- Li, N.; Su, J.; Xu, Z.; Li, D.-P.; Liu, Z.-T. Theoretical and experimental investigation on structural and electronic properties of Al/O/Al, O-doped WS 2. J. Phys. Chem. Solids 2016, 89, 84–88. [Google Scholar] [CrossRef]
- Abbas, O.A.; Lewis, A.H.; Aspiotis, N.; Huang, C.-C.; Zeimpekis, I.; Hewak, D.W.; Sazio, P.; Mailis, S. Laser printed two-dimensional transition metal dichalcogenides. Sci. Rep. 2021, 11, 5211. [Google Scholar] [CrossRef] [PubMed]
- Coehoorn, R.; Haas, C.; Dijkstra, J.; Flipse, C.J.F.; de Groot, R.A.; Wold, A. Electronic structure of MoSe2, MoS2, and WSe2. I. Band-structure calculations and photoelectron spectroscopy. Phys. Rev. B 1987, 35, 6195–6202. [Google Scholar] [CrossRef] [Green Version]
- Perea-López, N.; Elías, A.L.; Berkdemir, A.; Castro-Beltran, A.; Gutiérrez, H.R.; Feng, S.; Lv, R.; Hayashi, T.; López-Urías, F.; Ghosh, S. Photosensor device based on few-layered WS2 films. Adv. Funct. Mater. 2013, 23, 5511–5517. [Google Scholar] [CrossRef]
- Berkdemir, A.; Gutiérrez, H.R.; Botello-Méndez, A.R.; Perea-López, N.; Elías, A.L.; Chia, C.-I.; Wang, B.; Crespi, V.H.; López-Urías, F.; Charlier, J.-C. Identification of individual and few layers of WS2 using Raman Spectroscopy. Sci. Rep. 2013, 3, 1755. [Google Scholar] [CrossRef] [Green Version]
- Cesano, F.; Bertarione, S.; Piovano, A.; Agostini, G.; Rahman, M.M.; Groppo, E.; Bonino, F.; Scarano, D.; Lamberti, C.; Bordiga, S.; et al. Model oxide supported MoS2 HDS catalysts: Structure and surface properties. Catal. Sci. Technol. 2011, 1, 123–136. [Google Scholar] [CrossRef]
- Gutiérrez, H.R.; Perea-López, N.; Elías, A.L.; Berkdemir, A.; Wang, B.; Lv, R.; López-Urías, F.; Crespi, V.H.; Terrones, H.; Terrones, M. Extraordinary room-temperature photoluminescence in triangular WS2 monolayers. Nano Lett. 2013, 13, 3447–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, K.S.; Nagaraja, H. Effect of isoelectronic tungsten doping on molybdenum selenide nanostructures and their graphene hybrids for supercapacitors. Electrochim. Acta 2019, 302, 459–471. [Google Scholar] [CrossRef]
- Wu, X.; Li, H.; Yang, X.; Wang, X.; Miao, Z.; Zhou, P.; Zhao, J.; Zhou, J.; Zhuo, S. Reduced Graphene Oxide Hydrogel for High Energy Density Symmetric Supercapacitor with High Operation Potential in Aqueous Electrolyte. ChemElectroChem 2021, 8, 4353–4359. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.; Qian, F.; Han, T.Y.-J.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Supercapacitors Based on Three-Dimensional Hierarchical Graphene Aerogels with Periodic Macropores. Nano Lett. 2016, 16, 3448–3456. [Google Scholar] [CrossRef]
- Meng, X.; Lu, L.; Sun, C. Green synthesis of three-dimensional MnO2/graphene hydrogel composites as a high-performance electrode material for supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 16474–16481. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, G.Y.; Lim, J.; Kim, S.O. CNT–rGO Hydrogel-Integrated Fabric Composite Synthesized via an Interfacial Gelation Process for Wearable Supercapacitor Electrodes. ACS Omega 2021, 6, 19578–19585. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.Z.; Wu, Y.Q.; Shen, Y.K.; Zhang, C.; Xiong, Q.; Qin, H. Electrodepositing manganese oxide into a graphene hydrogel to fabricate an asymmetric supercapacitor. Electrochim. Acta 2018, 289, 158–167. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Li, N.; Liu, J.; Wang, M.; Deng, J.; Zhou, J.; Ma, Q. Catalytic hydrogenation of alkali lignin to bio-oil using fullerene-like vanadium sulfide. Energy Fuels 2015, 29, 255–261. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Fu, Q.; Pan, C. Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog. Nat. Sci. 2015, 25, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Khataee, A.; Eghbali, P.; Irani-Nezhad, M.H.; Hassani, A. Sonochemical synthesis of WS2 nanosheets and its application in sonocatalytic removal of organic dyes from water solution. Ultrason. Sonochemistry 2018, 48, 329–339. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Nagajyothi, P.C.; Devarayapalli, K.C.; Shim, J. Depositing reduced graphene oxide onto tungsten disulfide nanosheets via microwave irradiation: Confirmation of four-electron transfer-assisted oxygen reduction and methanol oxidation reaction. New J. Chem. 2020, 44, 10638–10647. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. Graphene Transforms Wide Band Gap ZnS to a Visible Light Photocatalyst. The New Role of Graphene as a Macromolecular Photosensitizer. ACS Nano 2012, 6, 9777–9789. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, Y.; Yu, B.; Fang, C.; Zhang, J. Metallic 1T-VS2 nanosheets featuring V2+ self-doping and mesopores towards an efficient hydrogen evolution reaction. Inorg. Chem. Front. 2019, 6, 3510–3517. [Google Scholar] [CrossRef]
- Fang, W.; Zhao, H.; Xie, Y.; Fang, J.; Xu, J.; Chen, Z. Facile hydrothermal synthesis of VS2/graphene nanocomposites with superior high-rate capability as lithium-ion battery cathodes. ACS Appl. Mater. Interfaces 2015, 7, 13044–13052. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Sun, X.; Wu, C.; Peng, L.; Lin, C.; Hu, S.; Yang, J.; Xie, Y. Metallic Few-Layered VS2 Ultrathin Nanosheets: High Two-Dimensional Conductivity for In-Plane Supercapacitors. J. Am. Chem. Soc. 2011, 133, 17832–17838. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Kundu, M. Carbon Free Nanostructured Plate like WS2 with Excellent Lithium Storage Properties. ChemistrySelect 2020, 5, 14183–14189. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, A.; Wang, C.; Wang, H.; Shen, Y.; Tian, X. Bifunctional reduced graphene oxide/V2O5 composite hydrogel: Fabrication, high performance as electromagnetic wave absorbent and supercapacitor. ChemPhysChem 2014, 15, 366–373. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors Performance Evaluation. Adv. Energy Mater. 2014, 5, 1401401. [Google Scholar] [CrossRef] [Green Version]
- Ratha, S.; Rout, C.S. Supercapacitor electrodes based on layered tungsten disulfide-reduced graphene oxide hybrids synthesized by a facile hydrothermal method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, J.; Liu, F.; Chang, X.; Chen, H.; Lin, H.; Han, S. Graphene-constructed flower-like porous Co(OH)2 with tunable hierarchical morphologies for supercapacitors. RSC Adv. 2016, 6, 16745–16750. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdum, S.S.; Thangarasu, S.; Oh, T.H. Three-Dimensional Ternary rGO/VS2/WS2 Composite Hydrogel for Supercapacitor Applications. Inorganics 2022, 10, 229. https://doi.org/10.3390/inorganics10120229
Magdum SS, Thangarasu S, Oh TH. Three-Dimensional Ternary rGO/VS2/WS2 Composite Hydrogel for Supercapacitor Applications. Inorganics. 2022; 10(12):229. https://doi.org/10.3390/inorganics10120229
Chicago/Turabian StyleMagdum, Sahil S., Sadhasivam Thangarasu, and Tae Hwan Oh. 2022. "Three-Dimensional Ternary rGO/VS2/WS2 Composite Hydrogel for Supercapacitor Applications" Inorganics 10, no. 12: 229. https://doi.org/10.3390/inorganics10120229
APA StyleMagdum, S. S., Thangarasu, S., & Oh, T. H. (2022). Three-Dimensional Ternary rGO/VS2/WS2 Composite Hydrogel for Supercapacitor Applications. Inorganics, 10(12), 229. https://doi.org/10.3390/inorganics10120229