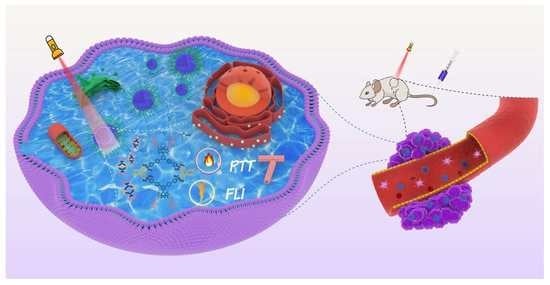
A Near-Infrared BODIPY-Based Rhomboidal Metallacycle for Imaging-Guided Photothermal Therapy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Metallacycle M
2.2. Fabrication and Characterization of F127/M NPs
2.3. Photothermal Properties of F127/M NPs
2.4. In Vitro Photonic Cytotoxicity and Antitumor Therapy
2.5. In Vivo Imaging and Antitumor Effect
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Zhou, Z.X.; Li, G.F.; Stang, P.J.; Yan, X.Z. Light-emitting self-assembled metallacages. Natl. Sci. Rev. 2021, 8, nwab045. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, C.; Stang, P.J. Soft Materials with Diverse Suprastructures via the Self-Assembly of Metal–Organic Complexes. Acc. Chem. Res. 2019, 52, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Sepehrpour, H.; Fu, W.; Sun, Y.; Stang, P.J. Biomedically Relevant Self-Assembled Metallacycles and Metallacages. J. Am. Chem. Soc. 2019, 141, 14005–14020. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Li, H.; Fan, Y.; He, T.; Qiu, H.; Yin, S. Metallacycle/Metallacage-Cored Fluorescent Supramolecular Assemblies with Aggregation-Induced Emission Properties. Adv. Optical Mater. 2020, 8, 1902190. [Google Scholar] [CrossRef]
- Li, B.; He, T.; Fan, Y.; Yuan, X.; Qiu, H.; Yin, S. Recent developments in the construction of metallacycle/metallacage-cored supramolecular polymers via hierarchical self-assembly. Chem. Commun. 2019, 55, 8036–8059. [Google Scholar] [CrossRef]
- Xu, L.; Shen, X.; Zhou, Z.; He, T.; Zhang, J.; Qiu, H.; Saha, M.L.; Yin, S.; Stang, P.J. Metallacycle-Cored Supramolecular Polymers: Fluorescence Tuning by Variation of Substituents. J. Am. Chem. Soc. 2018, 140, 16920–16924. [Google Scholar] [CrossRef]
- Chen, F.; Lin, X.; Li, Y.; Xu, D.; Qiu, H.; Yin, S. Metallacycle-crosslinked supramolecular polymers constructed by amino–YNE click reaction with enhanced mechanical properties. Supramol. Mater. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Chen, F.; Li, Y.; Qiu, H.-Y.; Zhang, J.-J.; Yin, S.-C. Supramolecular Polymer Networks with Enhanced Mechanical Properties: The Marriage of Covalent Polymer and Metallacycle. Chin. J. Chem. 2021, 39, 2731–2737. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, D.; Zhang, J.; Ni, R.; Xu, L.; He, T.; Lin, X.; Li, X.; Qiu, H.; Yin, S.; et al. Self-Healing Heterometallic Supramolecular Polymers Constructed by Hierarchical Assembly of Triply Orthogonal Interactions with Tunable Photophysical Properties. J. Am. Chem. Soc. 2019, 141, 17909–17917. [Google Scholar] [CrossRef]
- Hu, Y.X.; Hao, X.T.; Xu, L.; Xie, X.; Xiong, B.; Hu, Z.; Sun, H.; Yin, G.-Q.; Li, X.; Peng, H.; et al. Construction of supramolecular liquid-crystalline metallacycles for holographic storage of colored images. J. Am. Chem. Soc. 2020, 142, 6285–6294. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.L.; Sun, Y.; Lu, S.; Huo, H.; Tan, T.; Li, A.; Li, X.; Ungar, G.; Liu, F.; et al. Luminescent metallacycle-cored liquid crystals induced by metal coordination. Angew. Chem. Int. Ed. 2020, 59, 10143–10150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yin, S.; Zhang, J.; Zhou, Z.; Saha Manik, L.; Lu, C.; Stang Peter, J. Metallacycle-cored supramolecular assemblies with tunable fluorescence including white-light emission. Proc. Natl. Acad. Sci. USA 2017, 114, 3044–3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Saha, M.L.; Wang, M.; Zhou, Z.; Song, B.; Lu, C.; Yan, X.; Li, X.; Huang, F.; Yin, S.; et al. Multicomponent platinum(II) cages with tunable emission and amino acid sensing. J. Am. Chem. Soc. 2017, 139, 5067–5074. [Google Scholar] [CrossRef]
- Saha, M.L.; Yan, X.Z.; Stang, P.J. Photophysical properties of organoplatinum(II) compounds and derived self-assembled metallacycles and metallacages: Fluorescence and its applications. Acc. Chem. Res. 2016, 49, 2527–2539. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.X.; Chen, L.J.; Yang, H.B. Construction of multiferrocenyl metallacycles and metallacages via coordination-driven self-assembly: From structure to functions. Chem. Soc. Rev. 2020, 49, 3889–3919. [Google Scholar]
- Hong, T.; Zhang, Z.B.; Sun, Y.; Tao, J.-J.; Tang, J.-D.; Xie, C.; Wang, M.; Chen, F.; Xie, S.-S.; Li, S.; et al. Chiral metallacycles as catalysts for asymmetric conjugate addition of styrylboronic acids to α,β-Enones. J. Am. Chem. Soc. 2020, 142, 10244–10249. [Google Scholar] [CrossRef]
- Kaphan David, M.; Levin Mark, D.; Bergman Robert, G.; Raymond Kenneth, N.; Toste, F.D. A supramolecular microenvironment strategy for transition metal catalysis. Science 2015, 350, 1235–1238. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Li, Q.; Shi, B.; Ge, F.; Liu, Y.; Mao, Z.; Zhu, H.; Wang, S.; Yu, G.; Huang, F.; et al. Dual-Emissive Platinum(II) Metallacage with a Sensitive Oxygen Response for Imaging of Hypoxia and Imaging-Guided Chemotherapy. Angew. Chem. Int. Ed. 2020, 59, 20208–20214. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Li, Y.; Chen, Q.; Ni, Z.; Zhou, H.; Yu, J.; Qiu, H.; Yin, S. Amphiphilic rhomboidal metallacycles with aggregation-induced emission and aggregation-caused quenching luminogens for white-light emission and bioimaging. Mater. Chem. Front. 2022, 6, 633–643. [Google Scholar] [CrossRef]
- Yu, G.C.; Zhang, M.M.; Saha, M.L.; Mao, Z.; Chen, J.; Yao, Y.; Zhou, Z.; Liu, Y.; Gao, C.; Huang, F.; et al. Antitumor activity of a unique polymer that incorporates a fluorescent self-assembled metallacycle. J. Am. Chem. Soc. 2017, 139, 15940–15949. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.X.; Liu, J.P.; Rees, T.W.; Wang, H.; Li, X.; Chao, H.; Stang, P.J. Heterometallic Ru-Pt metallacycle for two-photon photodynamic therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 5664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, G.; Sun, Y.; Das, A.; Stang, P.J.; Yeon Lee, C. BODIPY based metal-organic macrocycles and frameworks: Recent therapeutic developments. Coord. Chem. Rev. 2022, 452, 214308. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, C.; Lu, S.; Wang, Z.; Liu, S.; Yu, X.; Li, X.; Sun, Y. Construction of emissive ruthenium(II) metallacycle over 1000 nm wavelength for in vivo biomedical applications. Nat. Commun. 2022, 13, 2009. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tong, Z.; Jin, L.; Ye, B.; Zhou, J.; Sun, Z.; Yang, H.; Hong, L.; Huang, F.; Wang, W.; et al. An NIR Discrete Metallacycle Constructed from Perylene Bisimide and Tetraphenylethylene Fluorophores for Imaging-Guided Cancer Radio-Chemotherapy. Adv. Mater. 2022, 34, 2106388. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Mack, J.; Yang, Y.C.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Zhao, X.; Fan, J.; Du, J.; Peng, X. Boron Dipyrromethene Nano-Photosensitizers for Anticancer Phototherapies. Small 2019, 15, 1804927. [Google Scholar] [CrossRef]
- Liu, M.; Ma, S.; She, M.; Chen, J.; Wang, Z.; Liu, P.; Zhang, S.; Li, J. Structural modification of BODIPY: Improve its applicability. Chin. Chem. Lett. 2019, 30, 1815–1824. [Google Scholar] [CrossRef]
- Bassan, E.; Gualandi, A.; Cozzi, P.G.; Ceroni, P. Design of BODIPY dyes as triplet photosensitizers: Electronic properties tailored for solar energy conversion, photoredox catalysis and photodynamic therapy. Chem. Sci. 2021, 12, 6607–6628. [Google Scholar] [CrossRef]
- Ito, F.; Nagai, T.; Ono, Y.; Yamaguchi, K.; Furuta, H.; Nagamura, T. Photophysical properties of 2-picolinoylpyrrole boron complex in solutions. Chem. Phys. Lett. 2007, 435, 283–288. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Squeo, B.M.; Gregoriou, V.G.; Avgeropoulos, A.; Baysec, S.; Allard, S.; Scherf, U.; Chochos, C.L. BODIPY-based polymeric dyes as emerging horizon materials for biological sensing and organic electronic applications. Prog. Polym. Sci. 2017, 71, 26–52. [Google Scholar] [CrossRef]
- Klfout, H.; Stewart, A.; Elkhalifa, M.; He, H. BODIPYs for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2017, 9, 39873–39889. [Google Scholar] [CrossRef]
- Ma, X.; Azeem, E.A.; Liu, X.L.; Cheng, Y.; Zhu, C. Synthesis and tunable chiroptical properties of chiral BODIPY-based D-π-A conjugated polymers. J. Mater. Chem. C 2014, 2, 1076–1084. [Google Scholar] [CrossRef]
- Bucher, L.; Desbois, N.; Harvey, P.D.; Sharma, G.D.; Gros, C.P. Porphyrins and BODIPY as Building Blocks for Efficient Donor Materials in Bulk Heterojunction Solar Cells. Solar RRL 2017, 1, 1700127. [Google Scholar] [CrossRef]
- Miao, J.; Wang, Y.; Liu, J.; Wang, L. Organoboron molecules and polymers for organic solar cell applications. Chem. Soc. Rev. 2022, 51, 153–187. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, K.; Kuang, G.; Masuda, T.; Zhang, A. Thermoresponsive Helical Poly(phenylacetylene)s. Macromolecules 2014, 47, 3288–3296. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.M.K.; Yoon, J. Recent developments ofBODIPY-based colorimetric and fluorescent probes for the detection of reactive oxygen/nitrogen species and cancer diagnosis. Coord. Chem. Rev. 2021, 439, 213936. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Ji, X.; Tao, Y.; Wang, J.; Zhao, W. BODIPY-Based Fluorescent Probes for Biothiols. Chem. Eur. J. 2020, 26, 4172–4192. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Bo, S.W.; Feng, T.; Qin, X.; Wan, Y.; Jiang, S.; Li, C.; Lin, J.; Wang, T.; Zhou, X.; et al. A versatile theranostic nanoemulsion for architecture-dependent multimodal imaging and dually augmented photodynamic therapy. Adv. Mater. 2019, 31, 1806444. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.C.; Cui, J.J.; Wu, F.Y.; Xiao, Q. A galactosidase-activatable fluorescent probe for detection of bacteria based on BODIPY. Molecules 2021, 26, 6072. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Kannadorai, R.K.; Yu, S.W.K.; Chang, Y.-T.; Wu, J. Push–pull type meso-ester substituted BODIPY near-infrared dyes as contrast agents for photoacoustic imaging. Org. Biomol. Chem. 2017, 15, 4531–4535. [Google Scholar] [CrossRef] [PubMed]
- Caruso, E.; Malacarne, M.C.; Marras, E.; Papa, E.; Bertato, L.; Banfi, S.; Gariboldi, M.B. New BODIPYs for photodynamic therapy (PDT): Synthesis and activity on human cancer cell lines. Biorg. Med. Chem. 2020, 28, 115737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, C.; Sun, T.T.; Xie, Z. Unadulterated BODIPY nanoparticles for biomedical applications. Coord. Chem. Rev. 2019, 390, 76–85. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Yan, Y.; El-Zohry, A.M.; Toffoletti, A.; Zhao, J.; Barbon, A.; Dick, B.; Mohammed, O.F.; Han, G. Elucidation of the Intersystem Crossing Mechanism in a Helical BODIPY for Low-Dose Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 16114–16121. [Google Scholar] [CrossRef]
- Yogo, T.; Urano, Y.; Ishitsuka, Y.; Maniwa, F.; Nagano, T. Highly Efficient and Photostable Photosensitizer Based on BODIPY Chromophore. J. Am. Chem. Soc. 2005, 127, 12162–12163. [Google Scholar] [CrossRef]
- Xi, D.M.; Xiao, M.; Cao, J.F.; Zhao, L.; Xu, N.; Long, S.; Fan, J.; Shao, K.; Sun, W.; Yan, X.; et al. NIR light-driving barrier-free group rotation in nanoparticles with an 88.3% photothermal conversion efficiency for photothermal therapy. Adv. Mater. 2020, 32, 1907855. [Google Scholar] [CrossRef]
- Lin, Y.; Yin, J.; Li, X.; Pan, C.; Kuang, G. Luminescent BODIPY-based Porous Organic Polymer for CO2 Adsorption. J. Wunan Univ. Technol. 2019, 34, 440–445. [Google Scholar] [CrossRef]
- Su, M.; Han, Q.; Yan, X.; Liu, Y.; Luo, P.; Zhai, W.; Zhang, Q.; Li, L.; Li, C. A Supramolecular Strategy to Engineering a Non-photobleaching and Near-Infrared Absorbing Nano-J-Aggregate for Efficient Photothermal Therapy. ACS Nano 2021, 15, 5032–5042. [Google Scholar] [CrossRef]
- Wang, X.; Lin, W.; Zhang, W.; Li, C.; Sun, T.; Chen, G.; Xie, Z. Amphiphilic redox-sensitive NIR BODIPY nanoparticles for dual-mode imaging and photothermal therapy. J. Colloid Interface Sci. 2019, 536, 208–214. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Zhao, W.; Zhao, W.; Li, F.; Xiao, K.; Yu, Q.; Liu, S.; Zhao, Q. Stable and Well-Organized Near-Infrared Platinum(II)–Acetylide-Based Metallacycles-Mediated Cancer Phototherapy. ACS Appl. Mater. Interfaces 2020, 12, 20180–20190. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Das, A.; Ghate, N.B.; Kim, T.; Ryu, J.Y.; Lee, J.; Mandal, N.; Lee, C.Y. Novel BODIPY-based Ru(ii) and Ir(iii) metalla-rectangles: Cellular localization of compounds and their antiproliferative activities. Chem. Commun. 2016, 52, 4274–4277. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; You, Y.; Hadiputra, R.; Jung, J.; Kang, D.-K.; Lee, C.Y. Heterometallic BODIPY-Based Molecular Squares Obtained by Self-Assembly: Synthesis and Biological Activities. ACS Omega 2019, 4, 13200–13208. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhang, Y.Z.; Yu, G.C.; Crawley, M.R.; Fulong, C.R.P.; Friedman, A.E.; Sengupta, S.; Sun, J.; Li, Q.; Huang, F.; et al. Highly emissive self-assembled BODIPY-platinum supramolecular triangles. J. Am. Chem. Soc. 2018, 140, 7730–7736. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, X.C.; Yu, J.L.; Fan, Y.; He, T.; Lu, S.; Li, X.; Qiu, H.; Yin, S. Amphiphilic rhomboidal organoplatinum(II) metallacycles with encapsulated doxorubicin for synergistic cancer therapy. ACS Appl. Bio Mater. 2020, 3, 8061–8068. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chen, R.; Cheng, X.J.; Marin, L. Bodipy-based chemosensors for highly sensitive and selective detection of Hg2+ ions. New J. Chem. 2018, 42, 19224–19231. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yu, J.; Li, W.; Fan, Y.; Li, Y.; Sun, Y.; Yin, S.; Stang, P.J. A Near-Infrared BODIPY-Based Rhomboidal Metallacycle for Imaging-Guided Photothermal Therapy. Inorganics 2022, 10, 80. https://doi.org/10.3390/inorganics10060080
Zhang J, Yu J, Li W, Fan Y, Li Y, Sun Y, Yin S, Stang PJ. A Near-Infrared BODIPY-Based Rhomboidal Metallacycle for Imaging-Guided Photothermal Therapy. Inorganics. 2022; 10(6):80. https://doi.org/10.3390/inorganics10060080
Chicago/Turabian StyleZhang, Jinjin, Jialin Yu, Wen Li, Yiqi Fan, Yang Li, Yan Sun, Shouchun Yin, and Peter J. Stang. 2022. "A Near-Infrared BODIPY-Based Rhomboidal Metallacycle for Imaging-Guided Photothermal Therapy" Inorganics 10, no. 6: 80. https://doi.org/10.3390/inorganics10060080
APA StyleZhang, J., Yu, J., Li, W., Fan, Y., Li, Y., Sun, Y., Yin, S., & Stang, P. J. (2022). A Near-Infrared BODIPY-Based Rhomboidal Metallacycle for Imaging-Guided Photothermal Therapy. Inorganics, 10(6), 80. https://doi.org/10.3390/inorganics10060080