Synthesis of a Heterometallic [Zn2Ca] Pinwheel Array Stabilized by Amide-Amide Synthons
Abstract
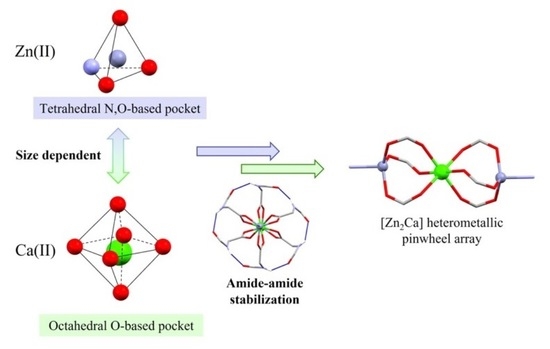
:1. Introduction
2. Experimental Section
2.1. Materials and General Methods
2.2. Synthesis of [Zn2Ca(μ-ACA)6(4-phpy)2]·EtOH (1)
2.3. X-ray Crystallographic Data
3. Results and Discussion
3.1. Synthesis and Characterization of 1
3.2. Structural Description and Hirshfeld Surface Analysis of 1C
3.3. CSD Study of Heterometallic [Zn2M] Pinwheel SBUs (M = S-Block Metal)
3.4. Photophysical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz, C.; Spodine, E.; Audebrand, N.; Venegas-Yazigi, D.; Paredes-García, V. Structural Versatility of 3d-CeIII Heterometallic Coordination Polymers Using CoII or CuII. Cryst. Growth Des. 2018, 18, 5155–5165. [Google Scholar] [CrossRef]
- Garden, J.A.; Saini, P.K.; Williams, C.K. Greater than the Sum of Its Parts: A Heterodinuclear Polymerization Catalyst. J. Am. Chem. Soc. 2015, 137, 15078–15081. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, L.; Zhu, G. Platinum-containing heterometallic complexes in cancer therapy: Advances and perspectives. Inorg. Chem. Front. 2022, 9, 2424–2453. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.; Lv, Y.; Dong, Y.; Hu, G.; Zhou, S.; Xu, Y. L- and D-[LnZn(IN)3(C2H4O2)]n (Ln = Eu, Sm, and Gd): Chiral Enantiomerically 3D 3d–4f Coordination Polymers Constructed by Interesting Butterfly-like Building. Inorg. Chem. 2016, 55, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Andruh, M. Heterotrimetallic complexes in molecular magnetism. Chem. Commun. 2018, 54, 3559–3577. [Google Scholar] [CrossRef]
- Buchwalter, P.; Rosé, J.; Braunstein, P. Multimetallic Catalysis Based on Heterometallic Complexes and Clusters. Chem. Rev. 2015, 115, 28–126. [Google Scholar] [CrossRef]
- Majumder, I.; Chakraborty, P.; Álvarez, R.; Gonzalez-Diaz, M.; Peláez, R.; Ellahioui, Y.; Bauza, A.; Frontera, A.; Zangrando, E.; Gómez-Ruiz, S.; et al. Bioactive Heterometallic CuII–ZnII Complexes with Potential Biomedical Applications. ACS Omega 2018, 3, 13343–13353. [Google Scholar] [CrossRef]
- Sakamoto, M. d-f Heteronuclear complexes: Synthesis, structures and physicochemical aspects. Coord. Chem. Rev. 2001, 219–221, 379–414. [Google Scholar] [CrossRef]
- Chipman, J.A.; Berry, J.F. Paramagnetic Metal–Metal Bonded Heterometallic Complexes. Chem. Rev. 2020, 120, 2409–2447. [Google Scholar] [CrossRef]
- Fromm, K.M. Chemistry of alkaline earth metals: It is not all ionic and definitely not boring! Coord. Chem. Rev. 2020, 408, 213193. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, L.; She, S.; Chen, Z.; Hu, B.; Li, Y. Two novel heterometallic CuII-SrII coordination polymers based on 3,5-pyrazoledicarboxylic acid: Synthesis, crystal structures and magnetic properties. Dalton Trans. 2011, 40, 4970. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; She, S.; Gao, Q.; Gao, D.; Wang, D.; Li, Y.; Liu, W.; Li, W. Synthesis, structures and properties of the first series of SrII–MII (M = Cu, Co, Ni and Zn) coordination polymers based on pyridine-2,5-dicarboxylic acid. CrystEngComm 2014, 16, 1091–1102. [Google Scholar] [CrossRef]
- Zang, Y.; Li, L.-K.; Zang, S.-Q. Recent development on the alkaline earth MOFs (AEMOFs). Coord. Chem. Rev. 2021, 440, 213955. [Google Scholar] [CrossRef]
- Fenton, H.; Tidmarsh, I.S.; Ward, M.D. Homonuclear and heteronuclear complexes of a four-armed octadentate ligand: Synthetic control based on matching ligand denticity with metal ion coordination preferences. Dalton Trans. 2009, 4199. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.-Y.; Cheng, J.-K.; Yao, Y.-G.; Zhang, J.; Wang, F. Alkaline earth metal ion doped Zn(II)-terephthalates. CrystEngComm 2012, 14, 4843. [Google Scholar] [CrossRef]
- Burguert, B.; Klatt, G.; Gallego, D.; Tan, G.; Driess, M. Unprecedent silicon(II)→calcium complexes with N-heterocyclic silylenes. Dalton Trans. 2015, 44, 639–644. [Google Scholar] [CrossRef]
- You, Z.; Wang, C.; Xiao, Y.; Guan, Q.; Li, J.; Xing, Y.; Gao, H.; Sun, L.; Bai, F. Integrated Photoresponsive Alkaline Earth Metal Coordination Networks: Synthesis, Topology, Photochromism and Photoluminescence Investigation. Chem.-A Eur. J. 2021, 27, 9605–9619. [Google Scholar] [CrossRef] [PubMed]
- Finelli, A.; Hérault, N.; Crochet, A.; Fromm, K.M. Compartmentalization of Alkaline-Earth Metals in Salen-Type Cu- and Ni-Complexes in Solution and in the Solid State. ACS Omega 2019, 4, 10231–10242. [Google Scholar] [CrossRef]
- Bo, Q.-B.; Zhang, Z.-W.; Miao, J.-L.; Wang, D.-Q.; Sun, G.-X. Novel metal–organic frameworks (MOFs) based on heterometallic nodes and 5-methylisophthalate linkers. CrystEngComm 2011, 13, 1765. [Google Scholar] [CrossRef]
- Akine, S.; Taniguchi, T.; Saiki, T.; Nabeshima, T. Ca2+-and Ba2+-Selective Receptors Based on Site-Selective Transmetalation of Multinuclear Polyoxime−Zinc(II) Complexes. J. Am. Chem. Soc. 2005, 127, 540–541. [Google Scholar] [CrossRef]
- Zou, R.; Zhong, R.; Han, S.; Xu, H.; Burrell, A.K.; Henson, N.; Cape, J.L.; Hickmott, D.D.; Timofeeva, T.V.; Larson, T.E.; et al. A Porous Metal−Organic Replica of α-PbO2 for Capture of Nerve Agent Surrogate. J. Am. Chem. Soc. 2010, 132, 17996–17999. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.-J.; Liu, G.-F.; Wang, B.-Q.; Lu, W.-B.; Zhai, Q.-G. Design of a heterometallic Zn/Ca-MOF decorated with alkoxy groups on the pore surface exhibiting high fluorescence sensing performance for Fe3+ and Cr2O72−. CrystEngComm 2020, 22, 4710–4715. [Google Scholar] [CrossRef]
- Yang, D.-L.; Zhang, X.; Yao, Y.-G.; Zhang, J. Structure versatility of coordination polymers constructed from a semirigid ligand and polynuclear metal clusters. CrystEngComm 2014, 16, 8047–8057. [Google Scholar] [CrossRef]
- Bo, Q.-B.; Wang, H.-Y.; Miao, J.-L.; Wang, D.-Q. Fluorescent Zn-based hetero-MOFs design via single metal site substitution. RSC Adv. 2012, 2, 11650. [Google Scholar] [CrossRef]
- Liu, L.-L.; Yu, Y.-Z.; Zhao, X.-J.; Wang, Y.-R.; Cheng, F.-Y.; Zhang, M.-K.; Shu, J.-J.; Liu, L. A robust Zn(II)/Na(II)-MOF decorated with [(OAc)2(H2O)2]n2n- anions for the luminescence sensing of copper ions based on the inner filter effect. Dalton Trans. 2018, 47, 7787–7794. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Du, P.; Yang, J.; Liu, Y.-Y.; Liu, X.-L.; Ma, J.-F. Two heterotrimetallic organic frameworks constructed using a functionalized Schiff base ligand: Syntheses, structures and visible photocatalytic activities for the degradation of chlorophenols. RSC Adv. 2016, 6, 98611–98619. [Google Scholar] [CrossRef]
- Ejarque, D.; Calvet, T.; Font-Bardia, M.; Pons, J. Construction of Zn(II) Linear Trinuclear Secondary Building Units from A Coordination Polymer Based on α-Acetamidocinnamic Acid and 4-Phenylpyridine. Molecules 2020, 25, 3615. [Google Scholar] [CrossRef] [PubMed]
- Ejarque, D.; Calvet, T.; Font-Bardia, M.; Pons, J. Amide-Driven Secondary Building Unit Structural Transformations between Zn(II) Coordination Polymers. Cryst. Growth Des. 2022, 22, 5012–5026. [Google Scholar] [CrossRef]
- Miroslaw, B.; Cristóvão, B.; Hnatejko, Z. Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization. Molecules 2018, 23, 1761. [Google Scholar] [CrossRef]
- Chao, M.-Y.; Li, Q.; Zhang, W.-H.; Young, D.J. Metal–organic frameworks of linear trinuclear cluster secondary building units: Structures and applications. Dalton Trans. 2021, 50, 12692–12707. [Google Scholar] [CrossRef]
- Mondal, S.; Dastidar, P. Designing Metallogelators Derived from NSAID-based Zn(II) Coordination Complexes for Drug-Delivery Applications. Chem.-An Asian J. 2020, 15, 3558–3567. [Google Scholar] [CrossRef] [PubMed]
- Smolková, R.; Zeleňák, V.; Smolko, L.; Sabolová, D.; Kuchár, J.; Gyepes, R. Novel Zn(II) complexes with non-steroidal anti-inflammatory ligand, flufenamic acid: Characterization, topoisomerase I inhibition activity, DNA and HSA binding studies. J. Inorg. Biochem. 2017, 177, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Sarma, R.J.; Baruah, J.B. Self-assembly of neutral dinuclear and trinuclear zinc-benzoate complexes. Inorg. Chem. Commun. 2006, 9, 1169–1172. [Google Scholar] [CrossRef]
- Konidaris, K.F.; Kaplanis, M.; Raptopoulou, C.P.; Perlepes, S.P.; Manessi-Zoupa, E.; Katsoulakou, E. Dinuclear versus trinuclear complex formation in zinc(II) benzoate/pyridyl oxime chemistry depending on the position of the oxime group. Polyhedron 2009, 28, 3243–3250. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Persistence of Vision Pty. Ltd. Persistence of Vision (TM) Raytracer; Persistence of Vision Pty. Ltd.: Williamstown, Victoria, Australia, 2004. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE. Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; Wiley Interscience: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of Infrared and Raman Spectroscopies to Characterize Metal-Organic Frameworks and Investigate Their Interaction with Guest Molecules. Chem. Rev. 2021, 121, 1286–1424. [Google Scholar] [CrossRef]
- Fleming, I.; Williams, D. Spectroscopic Methods in Organic Chemistry, 7th ed.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. 5. The Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Morse, P.M.; Girolami, G.S. Are d0 ML6 Complexes Always Octahedral? The X-ray Structure of Trigonal-Prismatic [Li(tmed)]2[ZrMe6]. J. Am. Chem. Soc. 1989, 111, 4114–4116. [Google Scholar] [CrossRef]
- Friese, J.C.; Krol, A.; Puke, C.; Kirschbaum, K.; Giolando, D.M. Trigonal prismatic vs octahedral coordination geometry: Syntheses and structural characterization of hexakis (arylthiolato) zirconate complexes. Inorg. Chem. 2000, 39, 1496–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Hou, H.; Meng, X.; Liu, Y.; Liu, Y.; Fan, Y. Ferrocenyl Functional Coordination Polymers Based on Mono-, Bi-, and Heterotrinuclear Organometallic Building Blocks: Syntheses, Structures, and Properties. Cryst. Growth Des. 2009, 9, 903–913. [Google Scholar] [CrossRef]
- Noh, K.; Ko, N.; Park, H.J.; Park, S.; Kim, J. Two porous metal–organic frameworks containing zinc–calcium clusters and calcium cluster chains. CrystEngComm 2014, 16, 8664–8668. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Clegg, W.; Hunt, P.A.; Straughan, B.P.; Mendiola, M.A. Preparation and Characterisation of Linear Mixed-metal Trinuclear Carboxylate Complexes of General Formula [M,M’(O2CR)6(C9H7N),]; Crystal Structure of [Zn2Ba(O2CCMe3)6. J. Chem. Soc. Dalton Trans. 1989, 1127–1131. [Google Scholar] [CrossRef]
- Rubtsova, I.K.; Melnikov, S.N.; Shmelev, M.A.; Nikolaevskii, S.A.; Yakushev, I.A.; Voronina, J.K.; Barabanova, E.D.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. Facile synthesis and structure elucidation of metal-organic frameworks with {ZnCa} and {Zn2Ca} metal cores. Mendeleev Commun. 2020, 30, 722–724. [Google Scholar] [CrossRef]
- Lemoine, P.; Viossat, B.; Dung, N.H.; Tomas, A.; Morgant, G.; Greenaway, F.T.; Sorenson, J.R.J. Synthesis, crystal structures, and anti-convulsant activities of ternary [ZnII(3,5-diisopropylsalicylate)2], [ZnII(salicylate)2] and [ZnII(aspirinate)2] complexes. J. Inorg. Biochem. 2004, 98, 1734–1749. [Google Scholar] [CrossRef] [PubMed]
- Clegg, W.; Harbron, D.R.; Straughan, B.P. Structures of the mixed-metal carboxylate base adducts [MgZn2(crotonate)6(4-vinylpyridine)2] and [MgCo2(crotonate)6(4-vinylpyridine)4]. Acta Cryst. 1991, C47, 267–270. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.; Guo, L.; Cao, J.; Li, W.; Liu, T.; Qiao, S.; Wang, B. A new porous heterometallic metal-organic framework for gas adsorption and luminescence sensing. Z. Für Anorg. Und Allg. Chem. 2021, 647, 1077–1082. [Google Scholar] [CrossRef]
- Kang, X.-P.; Zhu, L.-H.; Hu, Y.-S.; An, Z. Organically templated (3,8)-connected microporous heterometallic Zn(II)–Sr(II) coordination polymer. Inorg. Chem. Commun. 2013, 29, 11–13. [Google Scholar] [CrossRef]
- Clegg, W.; Little, I.R.; Straughan, B.P. Zinc Carboxylate Complexes: Structural Characterization of the Mixed-Metal Linear Trinuclear Complexes MZn2(crot)6(base)2 (M = Mn, Co, Ni, Zn, Cd, Mg, Ca, Sr; crot− = Crotonate(1-); Base = Quinoline, 6-Metyhlqui. Inorg. Chem. 1988, 27, 1916–1923. [Google Scholar] [CrossRef]
- Ma, L.-F.; Li, B.; Sun, X.-Y.; Wang, L.-Y.; Fan, Y.-T. Hydrothermal Syntheses and Characterizations of Three ZnII Coordination Polymers Tuned by pH Value and Base. Z. Für Anorg. Und Allg. Chem. 2010, 636, 1606–1611. [Google Scholar] [CrossRef]
- Rajak, R.; Saraf, M.; Mobin, S.M. Robust heterostructures of a bimetallic sodium–zinc metal–organic framework and reduced graphene oxide for high-performance supercapacitors. J. Mater. Chem. A 2019, 7, 1725–1736. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Zhou, X.-W.; Zhou, S.-R.; Tian, Y.-P.; Wu, J.-Y. A series of coordination polymers constructed from R-isophthalic acid (R=–SO3H, –NO2, and –OH) and N-donor ligands: Syntheses, structures and fluorescence properties. J. Solid State Chem. 2017, 245, 190–199. [Google Scholar] [CrossRef]
- Pramanik, A.; Fronczek, F.R.; Venkatraman, R.; Hossain, M.A. Hexa-μ-acetato-1:2κ4O,O′;1:2κ2O:O;2:3κ4O,O′;2:3κ2O:O-bis(4,4′-dimethyl-2,2′-bipyridine)-1κ2N,N′;3κ2N,N′-2-calcium-1,3-dizinc. Acta Cryst. 2013, E69, m643–m644. [Google Scholar] [CrossRef]
- Wu, Z.-F.; Huang, X.-Y. A series of Mg–Zn heterometallic coordination polymers: Synthesis, characterization, and fluorescence sensing for Fe3+, CS2, and nitroaromatic compounds. Dalton Trans. 2017, 46, 12597–12604. [Google Scholar] [CrossRef]
- Chen, H.; Fan, L.; Hu, T.; Zhang, X. 6s-3d {Ba3Zn4}–Organic Framework as an Effective Heterogeneous Catalyst for Chemical Fixation of CO2 and Knoevenagel Condensation Reaction. Inorg. Chem. 2021, 60, 3384–3392. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Introduction to Fluorescence. In Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2009; pp. 1–26. ISBN 978-1-4020-9002-8. [Google Scholar]
- Zhu, H.-B.; Zhao, J.; Kong, F.; Gou, S.-H.; Sun, Y.-M. Isostructural zinc (II) and cadmium (II) coordination complexes with 4-pyridin-4-yl-pyrimidine-2-sulfonate: Structure and fluorescent properties. J. Mol. Struct. 2009, 928, 95–98. [Google Scholar] [CrossRef]
- Alessi, P.J.; Carter, E.C.; Fairchild, M.D.; Hunt, R.W.G.; McCamy, C.S.; Kránicz, B.; Moore, J.R.; Morren, L.; Noobs, J.H.; Ohno, Y.; et al. CIE 15: Technical Report: Colorimetry, 3rd ed.; Carter, E.C., Ohno, Y., Pointer, M.R., Robertson, A.R., Sève, R., Schanda, J.D., Witt, K., Eds.; International Comimission on Illumination: Washington, DC, USA, 2004; ISBN 3-901-906-33-9. [Google Scholar]
- Song, Y.; Wang, B.; Liu, Y.; Guo, L.-J.; Cao, J.-J.; Li, W.-H.; Liu, T.; Qiao, S.; Guo, L.-D. Heterometallic trinuclear cluster-based microporous metal-organic framework with high adsorption selectivity of CO2 over N2. Inorg. Chem. Commun. 2020, 121, 108202. [Google Scholar] [CrossRef]
- Necefoglu, H.; Clegg, W.; Scott, A.J. A linear trinuclear CaZn2 complex with bridging benzoate ligands. Acta Cryst. Sect. E 2002, 58, m123–m124. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Zheng, F.; Ren, J.; Chen, G.; Qian, Y.; Huang, J. Two mixed-metal carboxylate–base adducts. Acta Cryst. 2000, C56, 1198–1200. [Google Scholar] [CrossRef]
- Escobedo-Martínez, C.; Lozada, M.C.; Gnecco, D.; Enriquez, R.G.; Soriano-García, M.; Reynolds, W.F. Acetate Bridged Trinuclear Zn, Ca and Mg Metal Complexes with 2- and 4-Substituted Pyridines. J. Chem. Crystallogr. 2012, 42, 794–802. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Zhang, S.-R.; Wang, W.; Xu, Y.-H.; Che, G.-B. A 3D metal–organic framework with dual-aerial-octahedral trinucleate building units: Synthesis, structure and fluorescence sensing properties. New J. Chem. 2018, 42, 14648–14654. [Google Scholar] [CrossRef]
- Gao, H.; Lou, X.; Li, Q.-T.; Du, W.-J.; Xu, C. Three new coordination polymers based on tripodal flexible ligand: Synthesis, structures and luminescent properties. Inorg. Chim. Acta 2014, 412, 46–51. [Google Scholar] [CrossRef]
- Hazra, S.; Das, L.K.; Bhattacharya, R.; Drew, M.G.B.; Ghosh, A. Variations of structures on changing the ratios of metal ions in rare Ca(II)–Zn(II) hetero-metallic self-assembled coordination polymers of hexamethylenetetramine and benzoate. J. Indian Chem. Soc. 2021, 98, 100097. [Google Scholar] [CrossRef]




| 1C | |
|---|---|
| CCDC | 2190605 |
| Empirical formula | C92H90CaN8O20Zn2 |
| Formula weight | 1798.53 |
| T (K) | 100(2) |
| Wavelength (Å) | 0.71073 |
| System, space group | Triclinic, P |
| Unit cell dimensions | |
| a (Å) | 11.6745(12) |
| b (Å) | 13.2064(13) |
| c (Å) | 15.5353(16) |
| α (°) | 67.641(3) |
| β (°) | 85.612(4) |
| γ (°) | 73.680(4) |
| V (Å3) | 2124.6(4) |
| Z | 1 |
| Dcalc (mg/m3) | 1.406 |
| μ (mm−1) | 0.703 |
| F (000) | 938 |
| Crystal size (mm3) | 0.243 × 0.115 × 0.048 |
| hkl ranges | −16 <= h <= 16 −17 <= k <= 18 0 <= l <= 22 |
| θ range (°) | 2.221 to 30.593 |
| Reflections collected/unique/[Rint] | 13,031/13,031/[Rint = 0.0885] |
| Completeness to θ (%) | 99.9 |
| Absorption correction | Semi-empirical from equivalents |
| Max. and min. transmission | 0.7461 and 0.6570 |
| Refinement method | Full-matrix least-squares on 2 |
| Data/Restrains/Parameters | 13,031/0/563 |
| Goodness-on-fit on 2 | 1.046 |
| Final R indices [I > 2σ(I)] | R1 = 0.0429, wR2 = 0.1153 |
| R indices (all data) | R1 = 0.0900, wR2 = 0.1406 |
| Extinction coefficient | n/a |
| Largest diff-peak and hole (e. Å−3) | 1.516 and −0.421 |
| Bond Lengths (Å) | ||||
| Zn(1)-O(1) | 1.9622(16) | Ca-O(2) | 2.2115(17) | |
| Zn(1)-O(4) | 1.9626(17) | Ca-O(5) | 2.2117(18) | |
| Zn(1)-O(7) | 1.9375(16) | Ca-O(8) | 2.2055(17) | |
| Zn(1)-N(4) | 2.0504(19) | |||
| Bond Angles (°) | ||||
| O(1)-Zn(1)-O(4) | 110.43(7) | O(2)-Ca-O(5)#1 | 93.98(7) | |
| O(1)-Zn(1)-N(4) | 102.87(7) | O(5)-Ca-O(5)#1 | 180.0 | |
| O(4)-Zn(1)-N(4) | 100.42(7) | O(8)-Ca-O(2) | 88.09(6) | |
| O(7)-Zn(1)-O(1) | 123.72(7) | O(8)-Ca-O(2)#1 | 91.91(6) | |
| O(7)-Zn(1)-O(4) | 117.39(8) | O(8)-Ca-O(5) | 89.50(7) | |
| O(7)-Zn(1)-N(4) | 96.16(7) | O(8)-Ca-O(5)#1 | 90.50(7) | |
| O(2)#1-Ca-O(2) | 180.0 | O(8)-Ca-O(8)#1 | 180.0 | |
| O(2)-Ca-O(5) | 86.02(7) | |||
| Twist Angles (°) | ||||
| O(2)#1-Cg(1)-Cg(2)-O(5)#1 | 58.08 | O(5)-Cg(1)-Cg(2)-O(8)#1 | 61.97 | |
| O(8)-Cg(1)-Cg(2)-O(2) | 59.95 | |||
| Intramolecular Interactions (Å) | ||||
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | >D-H···A (°) |
| N(1)-H(1N)···O(6) | 0.88 | 1.99 | 2.826(3) | 158 |
| N(2)-H(2N)···O(9) | 0.88 | 2.13 | 2.840(2) | 137 |
| N(3)-H(3)···O(3) | 0.88 | 2.17 | 2.892(3) | 140 |
| Intermolecular Interactions (Å) | ||||
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | >D-H···A (°) |
| O(1W)-H(1WO)···O(4) | 0.84 | 2.07 | 2.903(5) | 173 |
| O(2W)-H(2WO)···O(3) | 0.84 | 2.24 | 3.053(5) | 162 |
| C(14)-H(14)···O(1W) | 0.95 | 2.52 | 3.439(5) | 162 |
| C(30)-H(30)···O(1W) | 0.95 | 2.48 | 3.390(6) | 161 |
| C(40)-H(40)···O(2W) | 0.95 | 2.42 | 3.293(5) | 152 |
| C(18)-H(18)···Cg(3) | 0.95 | 3.05 | 3.791(5) | 136 |
| C(44)-H(44)···Cg(4) | 0.95 | 3.13 | 3.920(5) | 142 |
| #1: -x+1, -y+1, -z+1. Cg(1) = O(2)#1 O(5) O(8); Cg(2) = O(2) O(5)#1 O(8)#1; Cg(3) = C(4) C(5) C(6) C(7) C(8) C(9); Cg(4) = C(15) C(16) C(17) C(18) C(19) C(20) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejarque, D.; Calvet, T.; Font-Bardia, M.; Pons, J. Synthesis of a Heterometallic [Zn2Ca] Pinwheel Array Stabilized by Amide-Amide Synthons. Inorganics 2022, 10, 118. https://doi.org/10.3390/inorganics10080118
Ejarque D, Calvet T, Font-Bardia M, Pons J. Synthesis of a Heterometallic [Zn2Ca] Pinwheel Array Stabilized by Amide-Amide Synthons. Inorganics. 2022; 10(8):118. https://doi.org/10.3390/inorganics10080118
Chicago/Turabian StyleEjarque, Daniel, Teresa Calvet, Mercè Font-Bardia, and Josefina Pons. 2022. "Synthesis of a Heterometallic [Zn2Ca] Pinwheel Array Stabilized by Amide-Amide Synthons" Inorganics 10, no. 8: 118. https://doi.org/10.3390/inorganics10080118
APA StyleEjarque, D., Calvet, T., Font-Bardia, M., & Pons, J. (2022). Synthesis of a Heterometallic [Zn2Ca] Pinwheel Array Stabilized by Amide-Amide Synthons. Inorganics, 10(8), 118. https://doi.org/10.3390/inorganics10080118