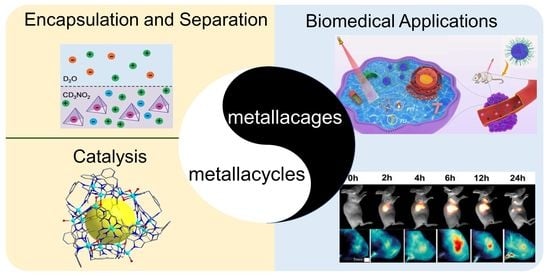
The Applications of Metallacycles and Metallacages
Abstract
:1. Introduction
2. Applications of Metallacycles and Metallacages
2.1. Encapsulation and Separation

2.2. Catalysis

2.3. Biomedical Applications
2.3.1. Drug Encapsulation and Delivery
2.3.2. Biological Recognition and Sensing
2.3.3. DNA Binding
2.3.4. Antibacterial Activity
2.3.5. Tumor Imaging and Treatment
2.4. Other Applications of Metallacycles and Metallacages
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; He, T.; Fan, Y.; Yuan, X.; Qiu, H.; Yin, S. Recent developments in the construction of metallacycle/metallacage-cored supramolecular polymers via hierarchical self-assembly. Chem. Commun. 2019, 55, 8036–8059. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-X.; Zhang, X.; Xu, L.; Yang, H.-B. Coordination-Driven Self-assembly of functionalized supramolecular metallacycles: Highlighted research during 2010–2018. Isr. J. Chem. 2019, 59, 184–196. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-X.; Chen, L.-J.; Yang, H.-B. Construction of multiferrocenyl metallacycles and metallacages via coordination-driven self-assembly: From structure to functions. Chem. Soc. Rev. 2015, 44, 2148–2167. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, Z.-J.; Liu, D.; Jin, G.-X. Multi-component coordination-driven self-assembly toward heterometallic macrocycles and cages. Coord. Chem. Rev. 2015, 293, 139–157. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.; Liu, J.; Stang, P.J. Recent developments in the construction and applications of platinum-based metallacycles and metallacages via coordination. Chem. Soc. Rev. 2020, 49, 3889–3919. [Google Scholar] [CrossRef]
- Sun, Y.; Tuo, W.; Stang, P.J. Metal-organic cycle-based multistage assemblies. Proc. Natl. Acad. Sci. USA 2022, 119, e2122398119. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.; Stang, P.J. Soft materials with diverse suprastructures via the self-assembly of metal-organic complexes. Acc. Chem. Res. 2019, 52, 802–817. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Shi, B.; Xing, H.; Sun, Y.; Lu, S.; Shangguan, L.; Li, X.; Huang, F.; Stang, P.J. Formation of planar chiral platinum triangles via pillar [5]arene for circularly polarized luminescence. J. Am. Chem. Soc. 2020, 142, 17340–17345. [Google Scholar] [CrossRef]
- Hong, T.; Zhang, Z.; Sun, Y.; Tao, J.-J.; Tang, J.-D.; Xie, C.; Wang, M.; Chen, F.; Xie, S.-S.; Li, S.; et al. Chiral metallacycles as catalysts for asymmetric conjugate addition of styrylboronic acids to α,β-enones. J. Am. Chem. Soc. 2020, 142, 10244–10249. [Google Scholar] [CrossRef]
- Lin, X.; Chen, F.; Yu, X.; Wang, H.; Qiu, H.; Li, Y.; Yin, S.; Stang, P.J. Phenylthiol-BODIPY-based supramolecular metallacycles for synergistic tumor chemo-photodynamic therapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2203994119. [Google Scholar] [CrossRef]
- Chang, X.; Zhou, Z.; Shang, C.; Wang, G.; Wang, Z.; Qi, Y.; Li, Z.-Y.; Wang, H.; Cao, L.; Li, X. Coordination-driven self-assembled metallacycles incorporating pyrene: Fluorescence mutability, tunability, and aromatic amine sensing. J. Am. Chem. Soc. 2019, 141, 1757–1765. [Google Scholar] [CrossRef]
- Acharyya, K.; Bhattacharyya, S.; Lu, S.; Sun, Y.; Mukherjee, P.S.; Stang, P.J. Emissive platinum(II) macrocycles as tunable cascade energy transfer scaffolds. Angew. Chem. Int. Ed. 2022, 61, e202200715. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Saha, M.L.; Stang, P.J. Hierarchical assemblies of supramolecular coordination complexes. Acc. Chem. Res. 2018, 51, 2047–2063. [Google Scholar] [CrossRef]
- Fink, D.; Orth, N.; Linseis, M.; Ivanović-Burmazović, I.; Winter, R.F. Ring size matters: Supramolecular isomerism in self-assembled redox-active tetra- and hexaruthenium macrocycles. Chem. Commun. 2020, 56, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rajasree, S.S.; Lee, G.Y.; Yu, J.; Tang, J.-H.; Ni, R.; Li, G.; Houk, K.N.; Deria, P.; Stang, P.J. Anthracene-triphenylamine-based platinum(II) metallacages as synthetic light-harvesting assembly. J. Am. Chem. Soc. 2021, 143, 2908–2919. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhu, B.; Shao, L.; Zhou, J.; Saha, M.L.; Shi, B.; Zhang, Z.; Hong, T.; Li, S.; Chen, X.; et al. Host-guest complexation-mediated codelivery of anticancer drug and photosensitizer for cancer photochemotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 6618–6623. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Cook, T.R.; Wang, S.-P.; Wu, J.; Li, S.; Stang, P.J. Self-assembly of chiral metallacycles and metallacages from a directionally adaptable BINOL-derived donor. J. Am. Chem. Soc. 2015, 137, 11896–11899. [Google Scholar] [CrossRef]
- Han, J.; Räder, A.F.B.; Reichart, F.; Aikman, B.; Wenzel, M.N.; Woods, B.; Weinmüller, M.; Ludwig, B.S.; Stürup, S.; Groothuis, G.M.M.; et al. Bioconjugation of supramolecular metallacages to integrin ligands for targeted delivery of cisplatin. Bioconjugate Chem. 2018, 29, 3856–3865. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yao, Y.; Wang, H.; Fu, W.X.; Chen, C.Y.; Saha, M.L.; Zhang, M.M.; Datta, S.; Zhou, Z.X.; Yu, H.X.; et al. Self-assembly of metallacages into multidimensional suprastructures with tunable emissions. J. Am. Chem. Soc. 2018, 140, 12819–12828. [Google Scholar] [CrossRef]
- Oldacre, A.N.; Friedman, A.E.; Cook, T.R. A self-assembled cofacial cobalt porphyrin prism for oxygen reduction catalysis. J. Am. Chem. Soc. 2017, 139, 1424–1427. [Google Scholar] [CrossRef]
- Paul, L.E.H.; Therrien, B.; Furrer, J. Interactions of arene ruthenium metallaprisms with human proteins. Org. Biomol. Chem. 2015, 13, 946–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, D.; Orth, N.; Ebel, V.; Gogesch, F.S.; Staiger, A.; Linseis, M.; Ivanović-Burmazović, I.; Winter, R.F. Self-assembled redox-active tetraruthenium macrocycles with large intracyclic cavities. Organometallics 2020, 39, 1861–1880. [Google Scholar] [CrossRef]
- Holloway, L.R.; Bogie, P.M.; Lyon, Y.; Ngai, C.; Miller, T.F.; Julian, R.R.; Hooley, R.J. Tandem reactivity of a self-assembled cage catalyst with endohedral acid groups. J. Am. Chem. Soc. 2018, 140, 8078–8081. [Google Scholar] [CrossRef] [PubMed]
- Malina, J.; Scott, P.; Brabec, V. Recognition of DNA/RNA bulges by antimicrobial and antitumor metallohelices. Dalton Trans. 2015, 44, 14656–14665. [Google Scholar] [CrossRef]
- Sunohara, H.; Koyamada, K.; Takezawa, H.; Fujita, M. An Ir3L2 complex with anion binding pockets: Photocatalytic E-Z isomerization via molecular recognition. Chem. Commun. 2021, 57, 9300–9302. [Google Scholar] [CrossRef]
- Gupta, G.; Denoyelle-Di-Muro, E.; Mbakidi, J.-P.; Leroy-Lhez, S.; Sol, V.; Therrien, B. Delivery of porphin to cancer cells by organometallic Rh(III) and Ir(III) metalla-cages. J. Organomet. Chem. 2015, 787, 44–50. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Z.; Qiao, Z.; Li, X.; Liu, Y.; Dong, J.; Jiang, J.; Cui, Y. Design and self-assembly of hexahedral coordination cages for cascade reactions. Nat. Commun. 2018, 9, 4423. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Gao, X.; Hang, X.; Zhu, X.; Han, H.; Liao, W.; Chen, W. Ultrafine Pt nanoclusters confined in a calixarene-Based {Ni24} coordination cage for high-efficient hydrogen evolution reaction. J. Am. Chem. Soc. 2016, 138, 16236–16239. [Google Scholar] [CrossRef]
- Jing, X.; He, C.; Yang, Y.; Duan, C. A metal-organic tetrahedron as a redox vehicle to encapsulate organic dyes for photocatalytic proton reduction. J. Am. Chem. Soc. 2015, 137, 3967–3974. [Google Scholar] [CrossRef]
- Deshmukh, M.S.; Mane, V.S.; Kumbhar, A.S.; Boomishankar, R. Light-driven hydrogen evolution from water by a tripodal silane based CoII6L18 octahedral cage. Inorg. Chem. 2017, 56, 13286–13292. [Google Scholar] [CrossRef]
- Dong, J.; Tan, C.; Zhang, K.; Liu, Y.; Low, P.J.; Jiang, J.; Cui, Y. Chiral NH-controlled supramolecular metallacycles. J. Am. Chem. Soc. 2017, 139, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-H.; Li, Y.; Wu, Q.; Wang, Z.; Hou, S.; Tang, K.; Sun, Y.; Wang, H.; Wang, H.; Lu, C.; et al. Single-molecule level control of host-guest interactions in metallocycle-C60 complexes. Nat. Commun. 2019, 10, 4599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.-M.; Fu, N.; Li, L.; Yuan, B.-Y.; Zhang, J.-H.; Li, Y.-X.; Yuan, L.-M. Homochiral metal-organic cage for gas chromatographic separations. Anal. Chem. 2018, 90, 9182–9188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, F.; Shen, X.; He, T.; Qiu, H.; Yin, S.; Stang, P.J. Self-healing metallacycle-cored supramolecular polymers based on a metal-salen complex constructed by orthogonal metal coordination and host-guest interaction with amino acid sensing. ACS Macro Lett. 2021, 10, 873–879. [Google Scholar] [CrossRef]
- Yin, C.; Xiao, P.; Liang, M.; Li, J.; Sun, Y.; Jiang, X.; Wu, W. Effects of iRGD conjugation density on the in vitro and in vivo properties of cylindrical polymer brushes. Biomater. Sci. 2022, 10, 3236–3244. [Google Scholar] [CrossRef]
- Yin, C.; Wang, R.; Sun, Y.; Li, S.; Zhang, X.; Gu, J.; Wu, W.; Jiang, X. The in vitro and in vivo properties of ringlike polymer brushes. Nano Today 2021, 41, 101293. [Google Scholar] [CrossRef]
- Rizzuto, F.J.; Nitschke, J.R. Stereochemical plasticity modulates cooperative binding in a CoII12L6 cuboctahedron. Nat. Chem. 2017, 9, 903–908. [Google Scholar] [CrossRef]
- Zhang, D.; Ronson, T.K.; Mosquera, J.; Martinez, A.; Guy, L.; Nitschke, J.R. Anion binding in water drives structural adaptation in an azaphosphatrane-functionalized FeII4L4 tetrahedron. J. Am. Chem. Soc. 2017, 139, 6574–6577. [Google Scholar] [CrossRef]
- Zhang, D.; Ronson, T.K.; Mosquera, J.; Martinez, A.; Nitschke, J.R. Selective anion extraction and recovery using a FeII4L4 cage. Angew. Chem. Int. Ed. 2018, 57, 3717–3721. [Google Scholar] [CrossRef] [Green Version]
- Tabuchi, R.; Takezawa, H.; Fujita, M. Selective confinement of rare-earth-metal hydrates by a capped metallo-cage under aqueous conditions. Angew. Chem. Int. Ed. 2022, 134, e202208866. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, X.; Lu, W.; Luo, D.; Zhu, X.W.; Li, X.; Zhou, X.P.; Li, D. Fine-tuning apertures of metal-organic cages: Encapsulation of carbon dioxide in solution and solid state. J. Am. Chem. Soc. 2019, 141, 11621–11627. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, H.; Shitozawa, K.; Fujita, M. Enhanced reactivity of twisted amides inside a molecular cage. Nat. Chem. 2020, 12, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, H.; Fujii, Y.; Murase, T.; Fujita, M. Electrophilic spirocyclization of a 2-biphenylacetylene via conformational fixing within a hollow-cage host. Angew. Chem. Int. Ed. 2022, 134, e202203970. [Google Scholar] [CrossRef]
- Tamura, Y.; Takezawa, H.; Fujita, M. A double-walled knotted cage for guest-adaptive molecular recognition. J. Am. Chem. Soc. 2020, 142, 5504–5508. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, H.; Wang, M.; Saha, M.L.; Zhou, Z.; Yan, X.; Wang, H.; Li, X.; Huang, F.; She, N.; et al. Platinum(II)-based convex trigonal-prismatic cages via coordination-driven self-assembly and C60 encapsulation. Inorg. Chem. 2017, 56, 12498–12504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, X.; Lin, S.; Wang, G.; Shang, C.; Wang, Z.; Liu, K.; Fang, Y.; Stang, P.J. Self-assembled perylene bisimide-cored trigonal prism as an electron-deficient host for C60 and C70 driven by “like dissolves like”. J. Am. Chem. Soc. 2020, 142, 15950–15960. [Google Scholar] [CrossRef]
- Struch, N.; Bannwarth, C.; Ronson, T.K.; Lorenz, Y.; Mienert, B.; Wagner, N.; Engeser, M.; Bill, E.; Puttreddy, R.; Rissanen, K.; et al. An octanuclear metallosupramolecular cage designed to exhibit spin-crossover behavior. Angew. Chem. Int. Ed. 2017, 56, 4930–4935. [Google Scholar] [CrossRef]
- Fujita, D.; Suzuki, R.; Fujii, Y.; Yamada, M.; Nakama, T.; Matsugami, A.; Hayashi, F.; Weng, J.-K.; Yagi-Utsumi, M.; Fujita, M. Protein stabilization and refolding in a gigantic self-assembled cage. Chem 2021, 7, 2672–2683. [Google Scholar] [CrossRef]
- Brenner, W.; Ronson, T.K.; Nitschke, J.R. Separation and selective formation of fullerene adducts within an MII8L6 cage. J. Am. Chem. Soc. 2017, 139, 75–78. [Google Scholar] [CrossRef]
- Hou, C.P.; Chen, X.L.; Huang, Z.J.; Lei, Y.; Xiao, L.M.; Huang, J.F.; Li, S.Y.; Liu, J.M. Robust heterogeneous photocatalyst for visible-light-driven hydrogen evolution promotion: Immobilization of a fluorescein dye-encapsulated metal-organic cage on TiO2. ACS Appl. Mater. Interfaces 2021, 13, 57230–57240. [Google Scholar] [CrossRef]
- Qin, S.; Lei, Y.; Huang, J.-F.; Lv, C.-Y.; Li, X.-A.; Su, P.-Y.; Liu, J.-M. Controllable visible-light-driven syngas evolution by a ternary titania hybrid sacrificial system with a photosensitive metal-organic PdII cage and ReI catalyst. ACS Sustain. Chem. Eng. 2022, 10, 8254–8264. [Google Scholar] [CrossRef]
- Kunz, V.; Schulze, M.; Schmidt, D.; Würthner, F. Trinuclear ruthenium macrocycles: Toward supramolecular water oxidation catalysis in pure water. ACS Energy Lett. 2017, 2, 288–293. [Google Scholar] [CrossRef]
- Schulze, M.; Kunz, V.; Frischmann, P.D.; Würthner, F. A supramolecular ruthenium macrocycle with high catalytic activity for water oxidation that mechanistically mimics photosystem II. Nat. Chem. 2016, 8, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Meza-Chincha, A.-L.; Lindner, J.O.; Schindler, D.; Schmidt, D.; Krause, A.-M.; Röhr, M.I.S.; Mitrić, R.; Würthner, F. Impact of substituents on molecular properties and catalytic activities of trinuclear Ru macrocycles in water oxidation. Chem. Sci. 2020, 11, 7654–7664. [Google Scholar] [CrossRef] [PubMed]
- Kunz, V.; Lindner, J.O.; Schulze, M.; Röhr, M.I.S.; Schmidt, D.; Mitrić, R.; Würthner, F. Cooperative water oxidation catalysis in a series of trinuclear metallosupramolecular ruthenium macrocycles. Energy Environ. Sci. 2017, 10, 2137–2153. [Google Scholar] [CrossRef]
- Schindler, D.; Meza-Chincha, A.-L.; Roth, M.; Würthner, F. Structure-activity relationship for di-up to tetranuclear macrocyclic ruthenium catalysts in homogeneous water oxidation. Chem. Eur. J. 2021, 27, 16938–16946. [Google Scholar] [CrossRef]
- Meza-Chincha, A.-L.; Schindler, D.; Natali, M.; Würthner, F. Effects of photosensitizers and reaction media on light-driven water oxidation with trinuclear ruthenium macrocycles. ChemPhotoChem 2021, 5, 173–183. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Long, Z.H.; Wang, X.Z.; Zhou, J.Y.; Wang, X.S.; Zhou, X.P.; Li, D. Cobalt-based metal-organic cages for visible-light-driven water oxidation. Inorg. Chem. 2021, 60, 10380–10386. [Google Scholar] [CrossRef]
- Lu, Y.L.; Song, J.Q.; Qin, Y.H.; Guo, J.; Huang, Y.H.; Zhang, X.D.; Pan, M.; Su, C.Y. A redox-active supramolecular Fe4L6 cage based on organic vertices with acid-base-dependent charge tunability for dehydrogenation catalysis. J. Am. Chem. Soc. 2022, 144, 8778–8788. [Google Scholar] [CrossRef]
- Qi, X.; Zhong, R.; Chen, M.; Sun, C.; You, S.; Gu, J.; Shan, G.; Cui, D.; Wang, X.; Su, Z. Single metal-organic cage decorated with an Ir(III) complex for CO2 photoreduction. ACS Catal. 2021, 11, 7241–7248. [Google Scholar] [CrossRef]
- Bhat, I.A.; Devaraj, A.; Howlader, P.; Chi, K.-W.; Mukherjee, P.S. Preparation of a chiral Pt12 tetrahedral cage and its use in catalytic Michael addition reaction. Chem. Commun. 2018, 54, 4814–4817. [Google Scholar] [CrossRef] [PubMed]
- Oldacre, A.N.; Crawley, M.R.; Friedman, A.E.; Cook, T.R. Tuning the activity of heterogeneous cofacial cobalt porphyrins for oxygen reduction electrocatalysis through self-assembly. Chem. Eur. J. 2018, 24, 10984–10987. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.M.; Xiong, P.; Azam, K.; Ni, Q.L.; Zeng, J.Q.; Gui, L.C.; Wang, X.J. A discrete tetrahedral indium cage as an efficient heterogeneous catalyst for the fixation of CO2 and the srecker reaction of ketones. Inorg. Chem. 2020, 59, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.M.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. Self-assembled tetrahedral hosts as supramolecular catalysts. Acc. Chem. Res. 2018, 51, 2447–2455. [Google Scholar] [CrossRef]
- Jing, X.; He, C.; Zhao, L.; Duan, C. Photochemical properties of host-guest supramolecular systems with structurally confined metal-organic capsules. Int. J. Antimicrob. Agents 2019, 52, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.A.; Jain, R.; Siddiqui, M.M.; Saini, D.K.; Mukherjee, P.S. Water-soluble Pd8L4 self-assembled molecular barrel as an aqueous carrier for hydrophobic curcumin. Inorg. Chem. 2017, 56, 5352–5360. [Google Scholar] [CrossRef]
- Mendez-Arroyo, J.; d’Aquino, A.I.; Chinen, A.B.; Manraj, Y.D.; Mirkin, C.A. Reversible and selective encapsulation of dextromethorphan and beta-estradiol using an asymmetric molecular capsule assembled via the weak-link approach. J. Am. Chem. Soc. 2017, 139, 1368–1371. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, H.; Bowers, D.J.; Gao, M.; Stilgenbauer, M.; Nielsen, F.; Shelley, J.T.; Zheng, Y.-R. Nanoparticles of metal-organic cages designed to encapsulate platinum-based anticancer agents. Dalton Trans. 2018, 47, 670–674. [Google Scholar] [CrossRef]
- Garci, A.; Mbakidi, J.-P.; Chaleix, V.; Sol, V.; Orhan, E.; Therrien, B. Tunable arene ruthenium metallaprisms to transport, shield, and release porphin in cancer cells. Organometallics 2015, 34, 4138–4146. [Google Scholar] [CrossRef]
- Mannancherril, V.; Therrien, B. Strategies toward the enhanced permeability and retention effect by increasing the molecular weight of arene ruthenium metallaassemblies. Inorg. Chem. 2018, 57, 3626–3633. [Google Scholar] [CrossRef]
- Gallardo-Villagrán, M.; Paulus, L.; Charissoux, J.-L.; Sutour, S.; Vergne-Salle, P.; Leger, D.Y.; Liagre, B.; Therrien, B. Evaluation of ruthenium-based assemblies as carriers of photosensitizers to treat rheumatoid arthritis by photodynamic therapy. Pharmaceutics 2021, 13, 2104. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-Q.; Fan, Y.-Z.; Wang, H.-P.; Teng, J.; Li, Y.-H.; Chen, C.-X.; Fenske, D.; Jiang, J.-J.; Su, C.-Y. Investigation of binding behavior between drug molecule 5-fluoracil and M4L4-type tetrahedral cages: Selectivity, capture, and release. Chem. Eur. J. 2017, 23, 3542–3547. [Google Scholar] [CrossRef] [PubMed]
- Woods, B.; Wenzel, M.N.; Williams, T.; Thomas, S.R.; Jenkins, R.L.; Casini, A. Exo-functionalized metallacages as host-guest systems for the anticancer drug cisplatin. Front. Chem. 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, D.; Lewis, J.E.M.; Crowley, J.D. Multicavity [PdnL4]2n+ cages with controlled segregated binding of different guests. J. Am. Chem. Soc. 2017, 139, 2379–2386. [Google Scholar] [CrossRef] [Green Version]
- Rizzuto, F.J.; Carpenter, J.P.; Nitschke, J.R. Multisite binding of drugs and natural products in an entropically favorable, heteroleptic receptor. J. Am. Chem. Soc. 2019, 141, 9087–9095. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, M.; Akita, M.; Hasegawa, T.; Hayashi, S.; Yoshizawa, M. A polyaromatic nanocapsule as a sucrose receptor in water. Sci. Adv. 2017, 3, e1701126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Li, S.; Zhang, Z.; Chen, L.; Zhang, M. A fluorescent platinum(II) metallacycle-cored supramolecular network formed by dynamic covalent bonds and its application in halogen ions and picric acid detection. Polym. Chem. 2020, 11, 254–258. [Google Scholar] [CrossRef]
- Zhang, M.; Saha, M.L.; Wang, M.; Zhou, Z.; Song, B.; Lu, C.; Yan, X.; Li, X.; Huang, F.; Yin, S.; et al. Multicomponent platinum(II) cages with tunable emission and amino acid sensing. J. Am. Chem. Soc. 2017, 139, 5067–5074. [Google Scholar] [CrossRef] [PubMed]
- Domarco, O.; Lötsch, D.; Schreiber, J.; Dinhof, C.; Van Schoonhoven, S.; García, M.D.; Peinador, C.; Keppler, B.K.; Berger, W.; Terenzi, A. Self-assembled Pt2L2 boxes strongly bind G-quadruplex DNA and influence gene expression in cancer cells. Dalton Trans. 2017, 46, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Garci, A.; Castor, K.J.; Fakhoury, J.; Do, J.-L.; Di Trani, J.; Chidchob, P.; Stein, R.S.; Mittermaier, A.K.; Friščić, T.; Sleiman, H. Efficient and rapid mechanochemical assembly of platinum(II) squares for guanine quadruplex targeting. J. Am. Chem. Soc. 2017, 139, 16913–16922. [Google Scholar] [CrossRef]
- Richards, A.D.; Rodger, A.; Hannon, M.J.; Bolhuis, A. Antimicrobial activity of an iron triple helicate. Int. J. Antimicrob. Agents 2009, 33, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Howson, S.E.; Bolhuis, A.; Brabec, V.; Clarkson, G.J.; Malina, J.; Rodger, A.; Scott, P. Optically pure, water-stable metallo-helical ‘flexicate’ assemblies with antibiotic activity. Nat. Chem. 2012, 4, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qian, X.; Wang, K.; Su, M.; Haoyang, W.-W.; Jiang, X.; Brzozowski, R.; Wang, M.; Gao, X.; Li, Y.; et al. Supramolecular kandinsky circles with high antibacterial activity. Nat. Commun. 2018, 9, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, C.-H.; Wang, K.; Wang, M.; Yu, H.; Kandapal, S.; Brzozowski, R.; Xu, B.; Wang, M.; Lu, S.; et al. Assembling pentatopic terpyridine ligands with three types of coordination moieties into a giant supramolecular hexagonal prism: Synthesis, self-assembly, characterization, and sntimicrobial study. J. Am. Chem. Soc. 2019, 141, 16108–16116. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yan, X.; Xie, G.; Zhu, M.; Ju, X.; Stang, P.J.; Tian, Y.; Niu, Z. Membrane intercalation-enhanced photodynamic inactivation of bacteria by a metallacycle and TAT-decorated virus coat protein. Proc. Natl. Acad. Sci. USA 2019, 116, 23437–23443. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Ali, S.R.; Venkateswarulu, M.; Howlader, P.; Zangrando, E.; De, M.; Mukherjee, P.S. Self-assembled Pd12 coordination cage as photoregulated oxidase-like nanozyme. J. Am. Chem. Soc. 2020, 142, 18981–18989. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y.; Shi, X.L.; Phan, H.; Qu, H.; Hu, Y.X.; Yin, G.Q.; Zhao, X.L.; Li, X.P.; Xu, L.; Yu, Q.L.; et al. Efficient self-assembly of heterometallic triangular necklace with strong antibacterial activity. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Jeyakkumar, P.; Liang, Y.; Guo, M.; Lu, S.; Xu, D.; Li, X.; Guo, B.; He, G.; Chu, D.; Zhang, M. Emissive metallacycle-crosslinked supramolecular networks with tunable crosslinking densities for bacterial imaging and killing. Angew. Chem. Int. Ed. 2020, 59, 15199–15203. [Google Scholar] [CrossRef]
- Xu, Y.; Tuo, W.; Yang, L.; Sun, Y.; Li, C.; Chen, X.; Yang, W.; Yang, G.; Stang, P.J.; Sun, Y. Design of a metallacycle-based supramolecular photosensitizer for in vivo image-guided photodynamic inactivation of bacteria. Angew. Chem. Int. Ed. 2022, 134, e202110048. [Google Scholar]
- Xu, Y.; Li, C.; Ma, X.; Tuo, W.; Tu, L.; Li, X.; Sun, Y.; Stang, P.J.; Sun, Y. Long wavelength-emissive Ru(II) metallacycle-based photosensitizer assisting in vivo bacterial diagnosis and antibacterial treatment. Proc. Natl. Acad. Sci. USA 2022, 119, e2209904119. [Google Scholar] [CrossRef]
- Orhan, E.; Garci, A.; Riedel, T.; Soudani, M.; Dyson, P.J.; Therrien, B. Cytotoxic double arene ruthenium metalla-cycles that overcome cisplatin resistance. J. Organomet. Chem. 2016, 803, 39–44. [Google Scholar] [CrossRef]
- Orhan, E.; Garci, A.; Riedel, T.; Dyson, P.J.; Therrien, B. Cytotoxicity of arene ruthenium metalla-rectangles incorporating bis-pyridyl diimide linkers. J. Organomet. Chem. 2016, 815, 53–58. [Google Scholar] [CrossRef]
- Gupta, G.; Oggu, G.S.; Nagesh, N.; Bokara, K.K.; Therrien, B. Anticancer activity of large metalla-assemblies built from half-sandwich complexes. CrystEngComm 2016, 18, 4952–4957. [Google Scholar] [CrossRef]
- Tuo, W.; Xu, Y.; Fan, Y.; Li, J.; Qiu, M.; Xiong, X.; Li, X.; Sun, Y. Biomedical applications of Pt(II) metallacycle/metallacage-based agents: From mono-chemotherapy to versatile imaging contrasts and theranostic platforms. Coord. Chem. Rev. 2021, 443, 214017. [Google Scholar] [CrossRef]
- Bhowmick, S.; Jana, A.; Singh, K.; Gupta, P.; Gangrade, A.; Mandal, B.B.; Das, N. Coordination-driven self-assembly of ionic irregular hexagonal metallamacrocycles via an organometallic clip and their cytotoxicity potency. Inorg. Chem. 2018, 57, 3615–3625. [Google Scholar] [CrossRef]
- Jana, A.; Bhowmick, S.; Kumar, S.; Singh, K.; Garg, P.; Das, N. Self-assembly of Pt(II) based nanoscalar ionic hexagons and their anticancer potencies. Inorg. Chim. Acta 2019, 484, 19–26. [Google Scholar] [CrossRef]
- Datta, S.; Misra, S.K.; Saha, M.L.; Lahiri, N.; Louie, J.; Pan, D.; Stang, P.J. Orthogonal self-assembly of an organoplatinum(II) metallacycle and cucurbit[8]uril that delivers curcumin to cancer cells. Proc. Natl. Acad. Sci. USA 2018, 115, 8087–8092. [Google Scholar] [CrossRef] [Green Version]
- Ji, T.; Xia, L.; Zheng, W.; Yin, G.-Q.; Yue, T.; Li, X.; Zhang, W.; Zhao, X.-L.; Yang, H.-B. Porphyrin-functionalized coordination star polymers and their potential applications in photodynamic therapy. Polym. Chem. 2019, 10, 6116–6121. [Google Scholar] [CrossRef]
- Wang, X.; Su, Q.; Zhang, Z.; Yang, J.; Zhang, Y.; Zhang, M. Biotinylated platinum(II) metallacage towards targeted cancer theranostics. Chem. Commun. 2020, 56, 8460–8463. [Google Scholar] [CrossRef]
- Gupta, G.; Das, A.; Park, K.C.; Tron, A.; Kim, H.; Mun, J.; Mandal, N.; Chi, K.-W.; Lee, C.Y. Self-assembled novel BODIPY-based palladium supramolecules and their cellular localization. Inorg. Chem. 2017, 56, 4615–4621. [Google Scholar] [CrossRef]
- Gupta, G.; You, Y.; Hadiputra, R.; Jung, J.; Kang, D.-K.; Lee, C.Y. Heterometallic BODIPY-based molecular squares obtained by self-assembly: Synthesis and biological activities. ACS Omega 2019, 4, 13200–13208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajibola Adeyemo, A.; Shettar, A.; Bhat, I.A.; Kondaiah, P.; Mukherjee, P.S. Self-assembly of discrete RuII8 molecular cages and their in vitro anticancer activity. Inorg. Chem. 2017, 56, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Li, X.; Shi, Y.; Ding, R.; Teng, M.; Zhang, P.; Cao, C.; Stang, P.J. Self-assembled ruthenium(II) metallacycles and metallacages with imidazole-based ligands and their in vitro anticancer activity. Proc. Natl. Acad. Sci. USA 2019, 116, 4090–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.C.; Zhang, M.M.; Saha, M.L.; Mao, Z.W.; Chen, J.; Yao, Y.; Zhou, Z.J.; Liu, Y.J.; Gao, C.Y.; Huang, F.H.; et al. Antitumor activity of a unique polymer that incorporates a fluorescent self-assembled metallacycle. J. Am. Chem. Soc. 2017, 139, 15940–15949. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhou, Z.; Long, W.; Yan, Y.; Li, Y.; Fu, T.; Liu, Y.; Zhao, Z.; Tan, W.; Stang, P.J. Self-assembled Pt(II) metallacycles enable precise cancer combination chemotherapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2202255119. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Yang, J.; Shi, B.; Ye, B.; Wang, M.; Huang, F.; Stang, P.J. Polymeric nanoparticles integrated from discrete organoplatinum(II) metallacycle by stepwise post-assembly polymerization for synergistic cancer therapy. Chem. Mater. 2020, 32, 4564–4573. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, F.; Zhou, Z.; Li, C.; Pu, M.; Xu, Y.; Zhan, Y.; Lu, X.; Li, H.; Yang, G.; et al. Rhomboidal Pt(II) metallacycle-based NIR-II theranostic nanoprobe for tumor diagnosis and image-guided therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 1968–1973. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Li, Q.; Shi, B.; Ge, F.; Liu, Y.; Mao, Z.; Zhu, H.; Wang, S.; Yu, G.; Huang, F.; et al. Dual-emissive platinum(II) metallacage with a sensitive oxygen response for imaging of hypoxia and imaging-guided chemotherapy. Angew. Chem. Int. Ed. 2020, 59, 20208–20214. [Google Scholar] [CrossRef]
- Ding, Y.; Tong, Z.; Jin, L.; Ye, B.; Zhou, J.; Sun, Z.; Yang, H.; Hong, L.; Huang, F.; Wang, W.; et al. An NIR discrete metallacycle constructed from perylene bisimide and tetraphenylethylene fluorophores for imaging-guided cancer radio-chemotherapy. Adv. Mater. 2022, 34, 2106388. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, T.; Li, S.; Zhang, Z.; Lu, S.; Jeyakkumar, P.; Song, Z.; Li, X.; Yu, G.; Chu, D. Fluorescent metallacycle-cored amphiphilic nanoparticles formed by β-cyclodextrin-based host-guest interactions towards cancer theranostics. Chem. A Eur. J. 2020, 26, 13031–13038. [Google Scholar] [CrossRef]
- Simões, J.C.S.; Sarpaki, S.; Papadimitroulas, P.; Therrien, B.; Loudos, G. Conjugated photosensitizers for imaging and PDT in cancer research. J. Med. Chem. 2020, 63, 14119–14150. [Google Scholar] [CrossRef] [PubMed]
- Gaschard, M.; Nehzat, F.; Cheminel, T.; Therrien, B. Arene ruthenium metalla-assemblies with anthracene moieties for PDT applications. Inorganics 2018, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Gallardo-Villagrán, M.; Paulus, L.; Charissoux, J.-L.; Leger, D.Y.; Vergne-Salle, P.; Therrien, B.; Liagre, B. Ruthenium-based assemblies incorporating tetrapyridylporphyrin panels: A photosensitizer delivery strategy for the treatment of rheumatoid arthritis by photodynamic therapy. Dalton Trans. 2022, 51, 9673–9680. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, R.; Shi, Y.; Cai, Y.; Chen, J.; Sun, S.; Zhang, W.; Tang, R. 2D amphiphilic organoplatinum(II) metallacycles: Their syntheses, self-assembly in water and potential application in photodynamic therapy. Chem. Commun. 2018, 54, 8068–8071. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, J.; Huang, J.; Rees, T.W.; Wang, Y.; Wang, H.; Li, X.; Chao, H.; Stang, P.J. A self-assembled Ru-Pt metallacage as a lysosome-targeting photosensitizer for 2-photon photodynamic therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 20296–20302. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Zhou, Z.; Yang, H.; Shan, C.; Yu, H.; Wojtas, L.; Zhang, M.; Mao, Z.; Wang, M.; Stang, P.J. Self-assembly of porphyrin-containing metalla-assemblies and cancer photodynamic therapy. Inorg. Chem. 2020, 59, 7380–7388. [Google Scholar] [CrossRef]
- Li, W.-Y.; Zhao, C.-W.; Zhang, Y.-F.; Guan, Q.; Wan, J.-J.; Ma, J.-P.; Li, Y.-A.; Dong, Y.-B. A metal-organic cage-based nanoagent for enhanced photodynamic antitumor therapy. Chem. Commun. 2021, 57, 7954–7957. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.J.; Dong, F.; Jiang, S.T.; Yin, G.Q.; Li, X.; Tian, Y.; Yang, H.B. Light-controlled generation of singlet oxygen within a discrete dual-stage metallacycle for cancer therapy. J. Am. Chem. Soc. 2019, 141, 8943–8950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, J.; Li, W.; Fan, Y.; Li, Y.; Sun, Y.; Yin, S.; Stang, P.J. A near-infrared BODIPY-based rhomboidal metallacycle for imaging-guided photothermal therapy. Inorganics 2022, 10, 80. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Zhao, W.; Zhao, W.; Li, F.; Xiao, K.; Yu, Q.; Liu, S.; Zhao, Q. Stable and well-organized near-infrared platinum(II)-acetylide-based metallacycles-mediated cancer phototherapy. ACS Appl. Mater. Interfaces 2020, 12, 20180–20190. [Google Scholar] [CrossRef]
- Yu, G.; Yu, S.; Saha, M.L.; Zhou, J.; Cook, T.R.; Yung, B.C.; Chen, J.; Mao, Z.; Zhang, F.; Zhou, Z.; et al. A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat. Commun. 2018, 9, 4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, F.; Chen, Z.; Kim, W.Y.; Sharma, A.; Li, C.; Ouyang, Q.; Zhu, H.; Yang, G.; Sun, Y.; Kim, J.S. A nano-cocktail of an NIR-II emissive fluorophore and organoplatinum(II) metallacycle for efficient cancer imaging and therapy. Chem. Sci. 2019, 10, 7023–7028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Ding, F.; Chen, Z.; Zhang, R.; Li, C.; Xu, Y.; Zhang, Y.; Ni, R.; Li, X.; Yang, G.; et al. Melanin-dot-mediated delivery of metallacycle for NIR-II/photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 16729–16735. [Google Scholar] [CrossRef] [Green Version]
- Acharyya, K.; Bhattacharyya, S.; Sepehrpour, H.; Chakraborty, S.; Lu, S.; Shi, B.; Li, X.; Mukherjee, P.S.; Stang, P.J. Self-assembled fluorescent Pt(II) metallacycles as artificial light-harvesting systems. J. Am. Chem. Soc. 2019, 141, 14565–14569. [Google Scholar] [CrossRef]
- Zhu, J.L.; Xu, L.; Ren, Y.Y.; Zhang, Y.; Liu, X.; Yin, G.Q.; Sun, B.; Cao, X.; Chen, Z.; Zhao, X.L.; et al. Switchable organoplatinum metallacycles with high quantum yields and tunable fluorescence wavelengths. Nat. Commun. 2019, 10, 4285. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Li, M.; Que, W.; Stang, P.J. Self-assembly of metal-ion-responsive supramolecular coordination complexes and their photophysical properties. Dalton Trans. 2017, 46, 3120–3124. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Ou, B.; Jiang, S.-T.; Yin, G.-Q.; Chen, L.-J.; Xu, L.; Li, X.; Yang, H.-B. Cross-linked AIE supramolecular polymer gels with multiple stimuli-responsive behaviours constructed by hierarchical self-assembly. Polym. Chem. 2018, 9, 2021–2030. [Google Scholar] [CrossRef]
- Zhang, D.; Ronson, T.K.; Güryel, S.; Thoburn, J.D.; Wales, D.J.; Nitschke, J.R. Temperature controls guest uptake and release from Zn4L4 tetrahedra. J. Am. Chem. Soc. 2019, 141, 14534–14538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, B.T.; Grommet, A.B.; Tron, A.; Georges, M.C.A.; Nitschke, J.R. Heat engine drives transport of an Fe(II)4L4 cage and cargo. Adv. Mater. 2020, 32, e1907241. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.H.; Sun, Y.; Gong, Z.L.; Li, Z.Y.; Zhou, Z.X.; Wang, H.; Li, X.P.; Saha, M.L.; Zhong, Y.W.; Stang, P.J. Temperature-responsive fluorescent organoplatinum(II) metallacycles. J. Am. Chem. Soc. 2018, 140, 7723–7729. [Google Scholar] [CrossRef]
- Lewis, J.E.M.; Gavey, E.L.; Cameron, S.A.; Crowley, J.D. Stimuli-responsive Pd2L4 metallosupramolecular cages: Towards targeted cisplatin drug delivery. Chem. Sci. 2012, 3, 778–784. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.; Zhang, F.; Jiang, S.; Stang, P.J. Pt Metallacage-based centimeter films for smart emissive poly(N-isopropylacrylamide) hydrogel devices. Mater. Chem. Phys. 2022, 277, 125544. [Google Scholar] [CrossRef]
- Gu, Y.; Alt, E.A.; Wang, H.; Li, X.; Willard, A.P.; Johnson, J.A. Photoswitching topology in polymer networks with metal-organic cages as crosslinks. Nature 2018, 560, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Qin, P.; Chai, Y.; Qu, W.-J.; Shangguan, L.; Lin, Q.; Zhang, Y.-M.; Sun, Y.; Huang, F.; Stang, P.J. An organoplatinum(II) metallacycle-based supramolecular amphiphile and its application in enzyme-responsive controlled release. Inorg. Chem. 2022, 61, 8090–8095. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, C.; Du, J.; Olenyuk, B.; Stang, P.J.; Sun, Y. The Applications of Metallacycles and Metallacages. Inorganics 2023, 11, 54. https://doi.org/10.3390/inorganics11020054
Yin C, Du J, Olenyuk B, Stang PJ, Sun Y. The Applications of Metallacycles and Metallacages. Inorganics. 2023; 11(2):54. https://doi.org/10.3390/inorganics11020054
Chicago/Turabian StyleYin, Changfeng, Jiaxing Du, Bogdan Olenyuk, Peter J. Stang, and Yan Sun. 2023. "The Applications of Metallacycles and Metallacages" Inorganics 11, no. 2: 54. https://doi.org/10.3390/inorganics11020054
APA StyleYin, C., Du, J., Olenyuk, B., Stang, P. J., & Sun, Y. (2023). The Applications of Metallacycles and Metallacages. Inorganics, 11(2), 54. https://doi.org/10.3390/inorganics11020054