Controlling Chiral Self-Sorting in Truxene-Based Self-Assembled Cages
Abstract
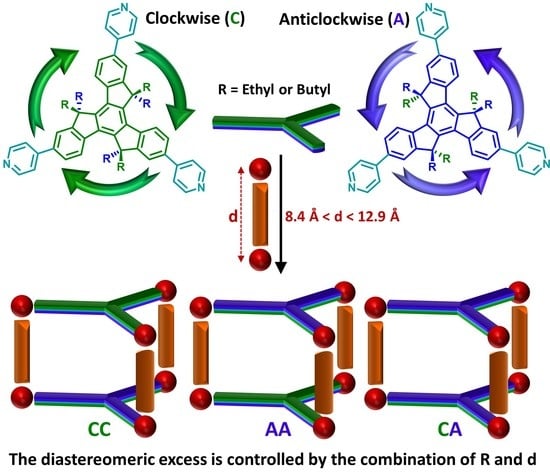
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation
3.3. Experimental Procedure and Characterization Data
3.3.1. Ligand LEt
3.3.2. Self-Assembly EtRu
3.3.3. Self-Assembly BuRh
3.4. Molecular Modelling
3.5. X-ray Crystallographic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Chen, C.; Liu, J.; Stang, P.J. Recent developments in the construction and applications of platinum-based metallacycles and metallacages via coordination. Chem. Soc. Rev. 2020, 49, 3889–3919. [Google Scholar] [CrossRef]
- Debata, N.B.; Tripathy, D.; Sahoo, H.S. Development of coordination driven self-assembled discrete spherical ensembles. Coord. Chem. Rev. 2019, 387, 273–298. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ronson, T.K.; Zou, Y.-Q.; Nitschke, J.R. Metal–organic cages for molecular separations—Nature Reviews Chemistry. Nat. Rev. Chem. 2021, 5, 168–182. [Google Scholar] [CrossRef]
- Grommet, A.B.; Feller, M.; Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 2020, 15, 256–271. [Google Scholar] [CrossRef]
- Percástegui, E.G.; Ronson, T.K.; Nitschke, J.R. Design and Applications of Water-Soluble Coordination Cages. Chem. Rev. 2020, 120, 13480–13544. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kannan, P.; Qiu, G. Cavity-based applications of metallo-supramolecular coordination cages (MSCCs). Org. Chem. Front. 2020, 7, 2842–2872. [Google Scholar] [CrossRef]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef]
- Gao, W.-X.; Zhang, H.-N.; Jin, G.-X. Supramolecular catalysis based on discrete heterometallic coordination-driven metallacycles and metallacages. Coord. Chem. Rev. 2019, 386, 69–84. [Google Scholar] [CrossRef]
- Rizzuto, F.J.; von Krbek, L.K.S.; Nitschke, J.R. Strategies for binding multiple guests in metal–organic cages. Nat. Rev. Chem. 2019, 3, 204–222. [Google Scholar] [CrossRef]
- Zhao, L.; Jing, X.; Li, X.; Guo, X.; Zeng, L.; He, C.; Duan, C. Catalytic properties of chemical transformation within the confined pockets of Werner-type capsules. Coord. Chem. Rev. 2019, 378, 151–187. [Google Scholar] [CrossRef]
- Casini, A.; Woods, B.; Wenzel, M. The Promise of Self-Assembled 3D Supramolecular Coordination Complexes for Biomedical Applications. Inorg. Chem. 2017, 56, 14715–14729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarra, S.; Wood, D.M.; Roberts, D.A.; Nitschke, J.R. Molecular containers in complex chemical systems. Chem. Soc. Rev. 2015, 44, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Amouri, H.; Desmarets, C.; Moussa, J. Confined Nanospaces in Metallocages: Guest Molecules, Weakly Encapsulated Anions, and Catalyst Sequestration. Chem. Rev. 2012, 112, 2015–2041. [Google Scholar] [CrossRef]
- Cai, K.; Zhang, L.; Astumian, R.D.; Stoddart, J.F. Radical-pairing-induced molecular assembly and motion. Nat. Rev. Chem. 2021, 5, 447–465. [Google Scholar] [CrossRef]
- Goeb, S.; Sallé, M. Electron-rich Coordination Receptors Based on Tetrathiafulvalene Derivatives: Controlling the Host-Guest Binding. Acc. Chem. Res. 2021, 54, 1043–1055. [Google Scholar] [CrossRef]
- Wezenberg, S.J. Light-switchable Metal-Organic Cages. Chem. Lett. 2020, 49, 609–615. [Google Scholar] [CrossRef]
- Chen, L.-J.; Yang, H.-B. Construction of Stimuli-Responsive Functional Materials via Hierarchical Self-Assembly Involving Coordination Interactions. Acc. Chem. Res. 2018, 51, 2699–2710. [Google Scholar] [CrossRef]
- Kim, T.Y.; Vasdev, R.A.S.; Preston, D.; Crowley, J.D. Strategies for Reversible Guest Uptake and Release from Metallosupramolecular Architectures. Chem. Eur. J. 2018, 24, 14878–14890. [Google Scholar] [CrossRef]
- Zhang, D.; Ronson, T.K.; Nitschke, J.R. Functional Capsules via Subcomponent Self-Assembly. Acc. Chem. Res. 2018, 51, 2423–2436. [Google Scholar] [CrossRef]
- Diaz-Moscoso, A.; Ballester, P. Light-responsive molecular containers. Chem. Commun. 2017, 53, 4635–4652. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.-X.; Yang, H.-B. Supramolecular transformations within discrete coordination-driven supramolecular architectures. Chem. Soc. Rev. 2016, 45, 2656–2693. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.-H.; Wang, Q.-C.; Zhang, Q.-W.; Ma, X.; Tian, H. Photoresponsive Host–Guest Functional Systems. Chem. Rev. 2015, 115, 7543–7588. [Google Scholar] [CrossRef]
- Trapp, O. Self-amplification of Enantioselectivity in Asymmetric Catalysis by Supramolecular Recognition and Stereodynamics. In Supramolecular Catalysis: New Directions and Developments; van Leeuwen, P.W.N.M., Raynal, M., Eds.; WILEY-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Zhang, L.; Liu, H.; Yuan, G.; Han, Y.-F. Chiral Coordination Metallacycles/Metallacages for Enantioselective Recognition and Separation. Chin. J. Chem. 2021, 39, 2273–2286. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xie, S.M.; Zi, M.; Yuan, L.M. Recent advances of application of porous molecular cages for enantioselective recognition and separation. J. Sep. Sci. 2020, 43, 134–149. [Google Scholar] [CrossRef]
- Pan, M.; Wu, K.; Zhang, J.-H.; Su, C.-Y. Chiral metal–organic cages/containers (MOCs): From structural and stereochemical design to applications. Coord. Chem. Rev. 2019, 378, 333–349. [Google Scholar] [CrossRef]
- Tan, C.; Chu, D.; Tang, X.; Liu, Y.; Xuan, W.; Cui, Y. Supramolecular Coordination Cages for Asymmetric Catalysis. Chem. Eur. J. 2019, 25, 662–672. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; He, C.; Meng, Q.; Duan, C. Asymmetric Catalysis within the Chiral Confined Space of Metal-Organic Architectures. Small 2019, 15, e1804770. [Google Scholar] [CrossRef]
- Chen, L.-J.; Yang, H.-B.; Shionoya, M. Chiral metallosupramolecular architectures. Chem. Soc. Rev. 2017, 46, 2555–2576. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Li, C.L.; Zuo, Y.; Zhao, Y.Q.; Zhang, S.D. Chiral Self-sorting in Cage-like Compounds. Chem. Lett. 2020, 49, 1356–1366. [Google Scholar] [CrossRef]
- Jędrzejewska, H.; Szumna, A. Making a Right or Left Choice: Chiral Self-Sorting as a Tool for the Formation of Discrete Complex Structures. Chem. Rev. 2017, 117, 4863–4899. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T.R.; Holstein, J.J.; Clever, G.H. Chiral Self-Discrimination and Guest Recognition in Helicene-Based Coordination Cages. Angew. Chem. Int. Ed. 2019, 58, 5562–5566. [Google Scholar] [CrossRef]
- Beaudoin, D.; Rominger, F.; Mastalerz, M. Chiral Self-Sorting of [2+3] Salicylimine Cage Compounds. Angew. Chem. Int. Ed. 2017, 56, 1244–1248. [Google Scholar] [CrossRef]
- Rota Martir, D.; Escudero, D.; Jacquemin, D.; Cordes, D.B.; Slawin, A.M.Z.; Fruchtl, H.A.; Warriner, S.L.; Zysman-Colman, E. Homochiral Emissive Λ8- and Δ8-[Ir8Pd4]16+ Supramolecular Cages. Chem. Eur. J. 2017, 23, 14358–14366. [Google Scholar] [CrossRef] [Green Version]
- Boer, S.A.; Turner, D.R. Self-selecting homochiral quadruple-stranded helicates and control of supramolecular chirality. Chem. Commun. 2015, 51, 17375–17378. [Google Scholar] [CrossRef]
- Maeda, C.; Kamada, T.; Aratani, N.; Osuka, A. Chiral self-discriminative self-assembling of meso–meso linked diporphyrins. Coord. Chem. Rev. 2007, 251, 2743–2752. [Google Scholar] [CrossRef]
- Lützen, A.; Hapke, M.; Griep-Raming, J.; Haase, D.; Saak, W. Synthesis and Stereoselective Self-Assembly of Double- and Triple-Stranded Helicates. Angew. Chem. Int. Ed. 2002, 41, 2086–2089. [Google Scholar] [CrossRef]
- Xu, C.; Lin, Q.; Shan, C.; Han, X.; Wang, H.; Wang, H.; Zhang, W.; Chen, Z.; Guo, C.; Xie, Y.; et al. Metallo-Supramolecular Octahedral Cages with Three Types of Chirality towards Spontaneous Resolution. Angew. Chem. 2022, 134, e202203099. [Google Scholar] [CrossRef]
- Masood, M.A.; Enemark, E.J.; Stack, T.D.P. Ligand Self-Recognition in the Self-Assembly of a [{Cu(L)}2]2+ Complex: The Role of Chirality. Angew. Chem. Int. Ed. 1998, 37, 928–932. [Google Scholar] [CrossRef]
- Arribas, C.S.; Wendt, O.F.; Sundin, A.P.; Carling, C.-J.; Wang, R.; Lemieux, R.P.; Wärnmark, K. Formation of an heterochiral supramolecular cage by diastereomer self-discrimination: Fluorescence enhancement and C60 sensing. Chem. Commun. 2010, 46, 4381–4383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weilandt, T.; Kiehne, U.; Schnakenburg, G.; Lützen, A. Diastereoselective self-assembly of dinuclear heterochiral metallosupramolecular rhombs in a self-discriminating process. Chem. Commun. 2009, 17, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Nava, P.; Colomban, C.; Martinez, A. Control and Transfer of Chirality Within Well-Defined Tripodal Supramolecular Cages. Front. Chem. 2020, 8, 599893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ronson, T.K.; Greenfield, J.L.; Brotin, T.; Berthault, P.; Léonce, E.; Zhu, J.-L.; Xu, L.; Nitschke, J.R. Enantiopure [Cs+/Xe⊂Cryptophane]⊂FeII4L4 Hierarchical Superstructures. J. Am. Chem. Soc. 2019, 141, 8339–8345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Wang, X.; Xuan, W.; Peng, P.; Li, Z.; Lu, R.; Wu, S.; Tian, Z.; Cao, X. Chiral separation and characterization of triazatruxene-based face-rotating polyhedra: The role of non-covalent facial interactions. Chem. Commun. 2018, 54, 4685–4688. [Google Scholar] [CrossRef]
- Qu, H.; Tang, X.; Wang, X.; Li, Z.; Huang, Z.; Zhang, H.; Tian, Z.; Cao, X. Chiral molecular face-rotating sandwich structures constructed through restricting the phenyl flipping of tetraphenylethylene. Chem. Sci. 2018, 9, 8814–8818. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fang, H.; Tranca, I.; Qu, H.; Wang, X.; Markvoort, A.J.; Tian, Z.; Cao, X. Elucidation of the origin of chiral amplification in discrete molecular polyhedra. Nat. Commun. 2018, 9, 488. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Peng, P.; Xuan, W.; Wang, Y.; Zhuang, Y.; Tian, Z.; Cao, X. Narcissistic chiral self-sorting of molecular face-rotating polyhedra. Org. Biomol. Chem. 2018, 16, 34–37. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, H.; Zhang, W.; Zhuang, Y.; Tian, Z.; Cao, X. Interconversion of molecular face-rotating polyhedra through turning inside out. Chem. Commun. (Camb.) 2017, 53, 8956–8959. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Yang, H.; Fang, H.; Chen, R.; Sun, Y.; Zheng, N.; Tan, K.; Lu, X.; Tian, Z.; et al. Assembled molecular face-rotating polyhedra to transfer chirality from two to three dimensions. Nat. Commun. 2016, 7, 12469. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.-L.; Zhang, D.; Ronson, T.K.; Wang, W.; Xu, L.; Yang, H.-B.; Nitschke, J.R. A Cavity-Tailored Metal-Organic Cage Entraps Gases Selectively in Solution and the Amorphous Solid State. Angew. Chem. Int. Ed. 2021, 60, 11789–11792. [Google Scholar] [CrossRef] [PubMed]
- Séjourné, S.; Labrunie, A.; Dalinot, C.; Benchohra, A.; Carré, V.; Aubriet, F.; Allain, M.; Sallé, M.; Goeb, S. Chiral Self-Sorting in Truxene-Based Metallacages. Inorganics 2020, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Bols, P.S.; Anderson, H.L. Shadow Mask Templates for Site-Selective Metal Exchange in Magnesium Porphyrin Nanorings. Angew. Chem. Int. Ed. 2018, 57, 7874–7877. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Telitel, S.; Contal, E.; Dumur, F.; Gigmes, D.; Bertin, D.; Nechab, M.; Graff, B.; Morlet-Savary, F.; et al. Polyaromatic Structures as Organo-Photoinitiator Catalysts for Efficient Visible Light Induced Dual Radical/Cationic Photopolymerization and Interpenetrated Polymer Networks Synthesis. Macromolecules 2012, 45, 4454–4460. [Google Scholar] [CrossRef]
- Kanibolotsky, A.L.; Berridge, R.; Skabara, P.J.; Perepichka, I.F.; Bradley, D.D.C.; Koeberg, M. Synthesis and Properties of Monodisperse Oligofluorene-Functionalized Truxenes: Highly Fluorescent Star-Shaped Architectures. J. Am. Chem. Soc. 2004, 126, 13695–13702. [Google Scholar] [CrossRef]
- Guan, J.; Xu, F.; Tian, C.; Pu, L.; Yuan, M.-S.; Wang, J. Tricolor Luminescence Switching by Thermal and Mechanical Stimuli in the Crystal Polymorphs of Pyridyl-substituted Fluorene. Chem. Asian J. 2019, 14, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Avram, L.; Frish, L. Diffusion NMR Spectroscopy in Supramolecular and Combinatorial Chemistry: An Old Parameter-New Insights. Angew. Chem. Int. Ed. 2005, 44, 520–554. [Google Scholar] [CrossRef]
- Mirtschin, S.; Slabon-Turski, A.; Scopelliti, R.; Velders, A.H.; Severin, K. A Coordination Cage with an Adaptable Cavity Size. J. Am. Chem. Soc. 2010, 132, 14004–14005. [Google Scholar] [CrossRef]
- Govindaswamy, P.; Linder, D.; Lacour, J.; Süss-Fink, G.; Therrien, B. Self-assembled hexanuclear arene ruthenium metallo-prisms with unexpected double helical chirality. Chem. Commun. 2006, 45, 4691–4693. [Google Scholar] [CrossRef] [Green Version]
- Barry, N.P.E.; Austeri, M.; Lacour, J.; Therrien, B. Highly Efficient NMR Enantiodiscrimination of Chiral Octanuclear Metalla-Boxes in Polar Solvent. Organometallics 2009, 28, 4894–4897. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.-S.; Fang, Q.; Liu, Z.-Q.; Guo, J.-P.; Chen, H.-Y.; Yu, W.-T.; Xue, G.; Liu, D.-S. Acceptor or Donor (Diaryl B or N) Substituted Octupolar Truxene: Synthesis, Structure, and Charge-Transfer-Enhanced Fluorescence. J. Org. Chem. 2006, 71, 7858–7861. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Furrer, J.; Therrien, B. In and Out of Cavity Interactions by Modulating the Size of Ruthenium Metallarectangles. Helv. Chim. Acta 2010, 93, 1313–1328. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-N.; Gao, W.-X.; Deng, Y.-X.; Lin, Y.-J.; Jin, G.-X. Stacking-interaction-induced host–guest chemistry and Borromean rings based on a polypyridyl ligand. Chem. Commun. 2018, 54, 1559–1562. [Google Scholar] [CrossRef] [PubMed]






| Alkyl Chain Length a (Å) | Metal-Metal Distance b (Å) | c = b − 2a (Å) | |
|---|---|---|---|
| BuRu | 5.0 | 8.4 | −1.6 |
| EtRu | 2.6 | 8.4 | 2.2 |
| BuRh | 5.0 | 12.9 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benchohra, A.; Séjourné, S.; Labrunie, A.; Miller, L.; Charbonneau, E.; Carré, V.; Aubriet, F.; Allain, M.; Sallé, M.; Goeb, S. Controlling Chiral Self-Sorting in Truxene-Based Self-Assembled Cages. Inorganics 2022, 10, 103. https://doi.org/10.3390/inorganics10070103
Benchohra A, Séjourné S, Labrunie A, Miller L, Charbonneau E, Carré V, Aubriet F, Allain M, Sallé M, Goeb S. Controlling Chiral Self-Sorting in Truxene-Based Self-Assembled Cages. Inorganics. 2022; 10(7):103. https://doi.org/10.3390/inorganics10070103
Chicago/Turabian StyleBenchohra, Amina, Simon Séjourné, Antoine Labrunie, Liam Miller, Enzo Charbonneau, Vincent Carré, Frédéric Aubriet, Magali Allain, Marc Sallé, and Sébastien Goeb. 2022. "Controlling Chiral Self-Sorting in Truxene-Based Self-Assembled Cages" Inorganics 10, no. 7: 103. https://doi.org/10.3390/inorganics10070103
APA StyleBenchohra, A., Séjourné, S., Labrunie, A., Miller, L., Charbonneau, E., Carré, V., Aubriet, F., Allain, M., Sallé, M., & Goeb, S. (2022). Controlling Chiral Self-Sorting in Truxene-Based Self-Assembled Cages. Inorganics, 10(7), 103. https://doi.org/10.3390/inorganics10070103