Direct Z-Scheme CoFe2O4-Loaded g-C3N4 Photocatalyst with High Degradation Efficiency of Methylene Blue under Visible-Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Photocatalyst
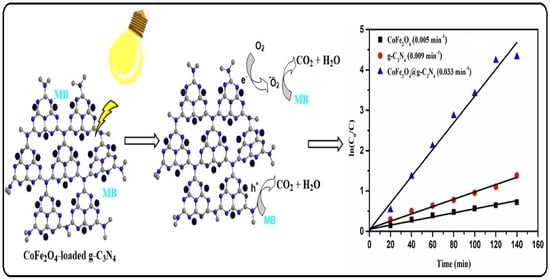
2.2. Photocatalytic Activity
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Photocatalysts
3.3. Characterization Techniques
3.4. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, X.; Ai, S.; Yang, D.; Zhao, L.; Ding, H. Synergistic photocatalytic and Fenton-like degradation of organic contaminants using peroxymonosulfate activated by CoFe2O4@g-C3N4 composite. Environ. Technol. 2021, 42, 2240–2253. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Eghbali, P.; Ekicibil, A.; Metin, Ö. Monodisperse cobalt ferrite nanoparticles assembled on mesoporous graphitic carbon nitride (CoFe2O4/mpg-C3N4): A magnetically recoverable nanocomposite for the photocatalytic degradation of organic dyes. J. Magn. Magn. Mater. 2018, 456, 400–412. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Abitorabi, M. Fabrication of novel magnetically separable nanocomposites using graphitic carbon nitride, silver phosphate and silver chloride and their applications in photocatalytic removal of different pollutants using visible-light irradiation. J. Colloid Interface Sci. 2016, 480, 218–231. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luan, J. Synthesis, Property Characterization and Photocatalytic Activity of the Novel Composite Polymer Polyaniline/Bi2SnTiO7. Molecules 2012, 17, 2752–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebrezgiabher, M.; Gebreslassie, G.; Gebretsadik, T.; Yeabyo, G.; Elemo, F.; Bayeh, Y.; Thomas, M.; Linert, W. A C-Doped TiO2/Fe3O4 Nanocomposite for Photocatalytic Dye Degradation under Natural Sunlight Irradiation. J. Compos. Sci. 2019, 3, 75. [Google Scholar] [CrossRef] [Green Version]
- Gebreslassie, G.; Bharali, P.; Gebremariam, G.; Sergawie, A.; Alemayehu, E. Graphitic Carbon Nitride with Extraordinary Photocatalytic Activity under Visible Light Irradiation; Springer International Publishing: Cham, Switzerland, 2021; Volume 385. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, H.; Li, X.; Fan, J.; Xiang, Q. Carbon—Graphitic Carbon Nitride Hybrids for Heterogeneous Photocatalysis. Small 2020, 17, 2005231. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Hou, L. A critical review of g-C3N4-based photocatalytic membrane for water purification. Chem. Eng. J. 2021, 412, 128663. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic carbon nitride (g-C3N4)–based metal-free photocatalysts for water splitting: A review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Chebanenko, M.I.; Tikhanova, S.M.; Nevedomskiy, V.N.; Popkov, V.I. Synthesis and Structure of ZnO-Decorated Graphitic Carbon Nitride (g-C3N4) with Improved Photocatalytic Activity under Visible Light. Inorganics 2022, 10, 249. [Google Scholar] [CrossRef]
- Farooq, N.; Luque, R.; Hessien, M.M.; Qureshi, A.M.; Sahiba, F.; Nazir, M.A.; ur Rehman, A. A comparative study of cerium- and ytterbium-based GO/g-C3N4/Fe2O3 composites for electrochemical and photocatalytic applications. Appl. Sci. 2021, 11, 9000. [Google Scholar] [CrossRef]
- Ismael, M.; Wark, M. Photocatalytic activity of CoFe2O4/g-C3N4 nanocomposite toward degradation of different organic pollutants and their inactivity toward hydrogen production: The role of the conduction band position. FlatChem 2022, 32, 100337. [Google Scholar] [CrossRef]
- Paul, A.; Dhar, S.S. Designing Cu2V2O7/CoFe2O4/g-C3N4 ternary nanocomposite: A high performance magnetically recyclable photocatalyst in the reduction of 4-nitrophenol to 4-aminophenol. J. Solid State Chem. 2020, 290, 121563. [Google Scholar] [CrossRef]
- He, W.; Liu, L.; Ma, T.; Han, H.; Zhu, J.; Liu, Y.; Fang, Z.; Yang, Z.; Guo, K. Controllable morphology CoFe2O4/g-C3N4 p-n heterojunction photocatalysts with built-in electric field enhance photocatalytic performance. Appl. Catal. B Environ. 2022, 306, 121107. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a Carbon Nitride Structure for Visible-Light Catalysis by Copolymerization. Angew. Chem. 2010, 49, 441–444. [Google Scholar] [CrossRef]
- Gebreslassie, G.; Bharali, P.; Chandra, U.; Sergawie, A.; Boruah, P.K.; Das, M.R.; Alemayehu, E. Novel g-C3N4/graphene/NiFe2O4 nanocomposites as magnetically separable visible light driven photocatalysts. J. Photochem. Photobiol. A Chem. 2019, 382, 111960. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Kumar, O.P.; Shahzad, K.; Nazir, M.A.; Farooq, N.; Malik, M.; Ahmad Shah, S.S.; ur Rehman, A. Photo-Fenton activated C3N4x/[email protected] dual s-scheme heterojunction towards degradation of organic pollutants. Opt. Mater. 2022, 126, 112199. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Huang, F.; Song, S.; Ai, G.; Xin, X.; Zhao, B.; Zheng, Y.; Zhang, Z. Recent Advances in g-C3N4-Based Materials and Their Application in Energy and Environmental Sustainability. Molecules 2023, 28, 432. [Google Scholar] [CrossRef]
- Gupta, N.K.; Gha, Y.; Kim, S.; Bae, J.; Kim, K.S. Photocatalytic Degradation of Organic Pollutants over MFe2O4 (M = Co, Ni, Cu, Zn) Nanoparticles at Neutral pH. Sci. Rep. 2020, 10, 4942. [Google Scholar] [CrossRef] [Green Version]
- Nife, O.; Holinsworth, B.S.; Mazumdar, D.; Sims, H.; Sun, Q.; Yurtisigi, M.K.; Sarker, S.K.; Gupta, A.; Butler, W.H.; Musfeldt, J.L. Chemical tuning of the optical band gap in spinel ferrites: CoFe2O4 vs. NiFe2O4. Appl. Phys. Lett. 2013, 103, 082406. [Google Scholar]
- He, H.; Lu, J. Highly photocatalytic activities of magnetically separable reduced graphene oxide-CoFe 2 O 4 hybrid nanostructures in dye photodegradation. Sep. Purif. Technol. 2017, 172, 374–381. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, H.; Sun, X.; Wang, X. Combination of cobalt ferrite and graphene: High-performance and recyclable visible-light photocatalysis. Appl. Catal. B Environ. 2012, 111, 280–287. [Google Scholar] [CrossRef]
- Huang, S.; Xu, Y.; Xie, M.; Xu, H.; He, M.; Xia, J.; Huang, L.; Li, H. Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light. Colloids Surf. A Physicochem. Eng. Asp. 2015, 478, 71–80. [Google Scholar] [CrossRef]
- Inbaraj, D.J.; Chandran, B.; Mangalaraj, C. Synthesis of CoFe2O4 and CoFe2O4/g-C3N4 nanocomposite via honey mediated sol-gel auto combustion method and hydrothermal method with enhanced photocatalytic and efficient Pb+2 adsorption property. Mater. Res. Express 2019, 6, 055501. [Google Scholar] [CrossRef]
- Yuan, C.; Cao, H.; Zhu, S.; Hua, H.; Hou, L. Core-shell ZnO/ZnFe2O4@C mesoporous nanospheres with enhanced lithium storage properties towards high-performance Li-ion batteries. J. Mater. Chem. A 2015, 3, 20389–20398. [Google Scholar] [CrossRef]
- Hou, L.; Lian, L.; Zhang, L.; Pang, G.; Yuan, C.; Zhang, X. Self-sacrifice template fabrication of hierarchical mesoporous bi-component-active ZnO/ZnFe2O4 sub-microcubes as superior anode towards high-performance lithium-ion battery. Adv. Funct. Mater. 2015, 25, 238–246. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric g-C 3 N 4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Zeng, M.; Zhao, G.; Xu, J.; Hu, W.; Wang, X. In Situ Synthesis of Water-Soluble Magnetic Graphitic Carbon Nitride Photocatalyst and Its Synergistic Catalytic Performance. ACS Appl. Mater. Interfaces 2013, 5, 12735–12743. [Google Scholar] [CrossRef]
- Anand, S.; Amaliya, A.P.; Janifer, M.A.; Pauline, S. Structural, morphological and dielectric studies of zirconium substituted CoFe2O4 nanoparticles. Mod. Electron. Mater. 2017, 3, 168–173. [Google Scholar] [CrossRef]
- Sabale, S.R. Studies on catalytic activity of MnFe2O4 and CoFe2O4MNPsas mediators in hemoglobin based biosensor. Mater. Today Proc. 2020, 23, 139–146. [Google Scholar] [CrossRef]
- Khan, M.; Pawar, H.; Kumari, M.; Patra, C.; Patel, G.; Dwivedi, U.K.; Rathore, D. Effect of concentration of SiC on physicochemical properties of CoFe2O4/SiC nanocomposites. J. Alloys Compd. 2020, 840, 155596. [Google Scholar] [CrossRef]
- Kumar, O.P.; Ahmad, M.; Nazir, M.A.; Anum, A.; Jamshaid, M.; Shah, S.S.A.; Rehman, A. Strategic combination of metal–organic frameworks and C3N4 for expeditious photocatalytic degradation of dye pollutants. Environ. Sci. Pollut. Res. 2022, 29, 35300–35313. [Google Scholar] [CrossRef] [PubMed]
- Renukadevi, S.; Jeyakumari, A.P. A one-pot microwave irradiation route to synthesis of CoFe2O4-g-C3N4 heterojunction catalysts for high visible light photocatalytic activity: Exploration of efficiency and stability. Diam. Relat. Mater. 2020, 109, 108012. [Google Scholar] [CrossRef]
- Hassani, A.; Çelikdağ, G.; Eghbali, P.; Sevim, M.; Karaca, S.; Metin, Ö. Heterogeneous sono-Fenton-like process using magnetic cobalt ferrite-reduced graphene oxide (CoFe2O4-rGO) nanocomposite for the removal of organic dyes from aqueous solution. Ultrason. Sonochem. 2018, 40, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Gebreslassie, G.; Bharali, P.; Chandra, U.; Sergawie, A.; Baruah, P.K.; Das, M.R.; Alemayehu, E. Hydrothermal Synthesis of g-C3N4/NiFe2O4 Nanocomposite and Its Enhanced Photocatalytic Activity. Appl. Organomet. Chem. 2019, 33, e5002. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Lakhera, S.K.; Narayanan, N.; Harish, S.; Hayakawa, Y.; Lee, B.K.; Neppolian, B. Environmentally Sustainable Synthesis of a CoFe2O4-TiO2/rGO Ternary Photocatalyst: A Highly Efficient and Stable Photocatalyst for High Production of Hydrogen (Solar Fuel). ACS Omega 2019, 4, 880–891. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]










| No. | Photocatalyst | Light Source | Target Dye | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|
| 1 | CoFe2O4/g-C3N4 | Sun light | MB | 98%/150 min | [28] |
| 2 | 41.4% CoFe2O4/g-C3N4-H2O2 | Xenon lamp | MB | 97.3%/180 min | [27] |
| 3 | CoFe2O4/g-C3N4-PMS | Halogen tungsten lamp | RhB | 96%/30 min | [1] |
| 4 | Direct Z-scheme CoFe2O4-loaded g-C3N4 | LED lamp | MB | 98.86%/140 min | This work |
| Photocatalyst | χ (eV) | Eg (eV) | ECB (eV) | EVB (eV) |
|---|---|---|---|---|
| CoFe2O4 | 5.81 | 1.30 | +0.66 | +1.96 |
| g-C3N4 | 4.73 | 2.65 | −1.09 | +1.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebreslassie, G.; Gebrezgiabher, M.; Lin, B.; Thomas, M.; Linert, W. Direct Z-Scheme CoFe2O4-Loaded g-C3N4 Photocatalyst with High Degradation Efficiency of Methylene Blue under Visible-Light Irradiation. Inorganics 2023, 11, 119. https://doi.org/10.3390/inorganics11030119
Gebreslassie G, Gebrezgiabher M, Lin B, Thomas M, Linert W. Direct Z-Scheme CoFe2O4-Loaded g-C3N4 Photocatalyst with High Degradation Efficiency of Methylene Blue under Visible-Light Irradiation. Inorganics. 2023; 11(3):119. https://doi.org/10.3390/inorganics11030119
Chicago/Turabian StyleGebreslassie, Gebrehiwot, Mamo Gebrezgiabher, Bin Lin, Madhu Thomas, and Wolfgang Linert. 2023. "Direct Z-Scheme CoFe2O4-Loaded g-C3N4 Photocatalyst with High Degradation Efficiency of Methylene Blue under Visible-Light Irradiation" Inorganics 11, no. 3: 119. https://doi.org/10.3390/inorganics11030119
APA StyleGebreslassie, G., Gebrezgiabher, M., Lin, B., Thomas, M., & Linert, W. (2023). Direct Z-Scheme CoFe2O4-Loaded g-C3N4 Photocatalyst with High Degradation Efficiency of Methylene Blue under Visible-Light Irradiation. Inorganics, 11(3), 119. https://doi.org/10.3390/inorganics11030119