Molten Salt Synthesis of Micro-Sized Hexagonally Shaped REMnO3 (RE = Y, Er, Tm, Yb)
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
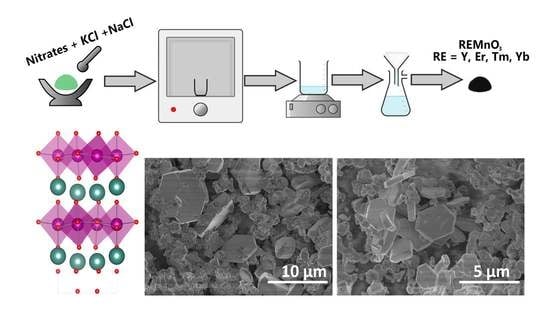
3.1. Synthesis
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lilienblum, M.; Lottermoser, T.; Manz, S.; Selbach, S.M.; Cano, A.; Fiebig, M. Ferroelectricity in the Multiferroic Hexagonal Manganites. Nat. Phys. 2015, 11, 1070–1073. [Google Scholar] [CrossRef]
- Das, H.; Wysocki, A.L.; Geng, Y.; Wu, W.; Fennie, C.J. Bulk Magnetoelectricity in the Hexagonal Manganites and Ferrites. Nat. Commun. 2014, 5, 2998. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, B. Hexagonal Manganites—(RMnO3): Class (I) Multiferroics with Strong Coupling of Magnetism and Ferroelectricity. Int. Sch. Res. Not. 2013, 2013, 497073. [Google Scholar] [CrossRef]
- Li, M.; Tan, H.; Duan, W. Hexagonal Rare-Earth Manganites and Ferrites: A Review of Improper Ferroelectricity, Magnetoelectric Coupling, and Unusual Domain Walls. Phys. Chem. Chem. Phys. 2020, 22, 14415–14432. [Google Scholar] [CrossRef] [PubMed]
- Manipatruni, S.; Nikonov, D.E.; Lin, C.-C.; Gosavi, T.A.; Liu, H.; Prasad, B.; Huang, Y.-L.; Bonturim, E.; Ramesh, R.; Young, I.A. Scalable Energy-Efficient Magnetoelectric Spin–Orbit Logic. Nature 2019, 565, 35–42. [Google Scholar] [CrossRef]
- Ferson, N.D.; Uhl, A.M.; Andrew, J.S. Piezoelectric and Magnetoelectric Scaffolds for Tissue Regeneration and Biomedicine: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2020, 68, 229–241. [Google Scholar] [CrossRef]
- Arya, G.; Yogiraj, J.; Negi, N.S.; Shah, J.; Kotnala, R.K. Observation of Enhanced Multiferroic, Magnetoelectric and Photocatalytic Properties in Sm-Co Codoped BiFeO3 Nanoparticles. J. Alloys Compd. 2017, 723, 983–994. [Google Scholar] [CrossRef]
- Mansour, S.F.; Imam, N.G.; Goda, S.; Abdo, M.A. Constructive Coupling between BiFeO3 and CoFe2O4; Promising Magnetic and Dielectric Properties. J. Mater. Res. Technol. 2020, 9, 1434–1446. [Google Scholar] [CrossRef]
- Lee, K.; Hajra, S.; Sahu, M.; Kim, H.J. Colossal Dielectric Response, Multiferroic Properties, and Gas Sensing Characteristics of the Rare Earth Orthoferrite LaFeO3 Ceramics. J. Alloys Compd. 2021, 882, 160634. [Google Scholar] [CrossRef]
- Huang, Z.J.; Cao, Y.; Sun, Y.Y.; Xue, Y.Y.; Chu, C.W. Coupling between the Ferroelectric and Antiferromagnetic Orders in YMnO3. Phys. Rev. B 1997, 56, 2623. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, G.; Gu, L.; Yao, Y.; Jin, C.; Wang, Y.; Duan, X.; Yu, R. Direct Observation of Multiferroic Vortex Domains in YMnO3. Sci. Rep. 2013, 3, 2741. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.M.; Lilienblum, M.; Delaney, K.T.; Kumagai, Y.; Fiebig, M.; Spaldin, N.A. Scaling Behavior and beyond Equilibrium in the Hexagonal Manganites. Phys. Rev. X 2012, 2, 041022. [Google Scholar] [CrossRef]
- van Aken, B.B.; Palstra, T.T.M.; Filippetti, A.; Spaldin, N.A. The Origin of Ferroelectricity in Magnetoelectric YMnO3. Nat. Mater. 2004, 3, 164–170. [Google Scholar] [CrossRef]
- Wang, S.F.; Yang, H.; Xian, T.; Liu, X.Q. Size-Controlled Synthesis and Photocatalytic Properties of YMnO3 Nanoparticles. Catal. Commun. 2011, 12, 625–628. [Google Scholar] [CrossRef]
- Han, A.; Zhao, M.; Ye, M.; Liao, J.; Zhang, Z.; Li, N. Crystal Structure and Optical Properties of YMnO3 Compound with High Near-Infrared Reflectance. Sol. Energy 2013, 91, 32–36. [Google Scholar] [CrossRef]
- Addabbo, T.; Bertocci, F.; Fort, A.; Gregorkiewitz, M.; Mugnaini, M.; Spinicci, R.; Vignoli, V. Gas Sensing Properties of YMnO3 Based Materials for the Detection of NOx and CO. Sens. Actuators B Chem. 2017, 244, 1054–1070. [Google Scholar] [CrossRef]
- Tian, M.; Liu, X.; Gong, A.; Zhang, S.; Wang, G.; Han, P.; Li, Y.; Lou, X.; Hao, X. Efficient Ultraviolet–Visible-near Infrared Self-Powered Photodetector Based on Hexagonal YMnO3-Based Ferroelectric Thin Film by Multiscale Polarity Structure Optimization. Chem. Eng. J. 2023, 452, 139040. [Google Scholar] [CrossRef]
- Wadati, H.; Okamoto, J.; Garganourakis, M.; Scagnoli, V.; Staub, U.; Yamasaki, Y.; Nakao, H.; Murakami, Y.; Mochizuki, M.; Nakamura, M. Origin of the Large Polarization in Multiferroic YMnO3 Thin Films Revealed by Soft-and Hard-X-Ray Diffraction. Phys. Rev. Lett. 2012, 108, 047203. [Google Scholar] [CrossRef]
- Ahmad, T.; Lone, I.H.; Ubaidullah, M. Structural Characterization and Multiferroic Properties of Hexagonal Nano-Sized YMnO3 Developed by a Low Temperature Precursor Route. RSC Adv. 2015, 5, 58065–58071. [Google Scholar] [CrossRef]
- Turut, A.; Coșkun, M.; Coșkun, F.M.; Polat, O.; Durmuș, Z.; Çağlar, M.; Efeoğlu, H. The Current-Voltage Characteristics of the Ferroelectric p-YMnO3 Thin Film/Bulk p-Si Heterojunction over a Broad Measurement Temperature Range. J. Alloys Compd. 2019, 782, 566–575. [Google Scholar] [CrossRef]
- Ren, P.; Fan, H.; Wang, X. Bulk Conduction and Nonlinear Behaviour in Multiferroic YMnO3. Appl. Phys. Lett. 2013, 103, 152905. [Google Scholar] [CrossRef]
- DiSalvo, F.J. Solid-State Chemistry: A Rediscovered Chemical Frontier. Science 1990, 247, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, T.; Song, S.; Ravi, M.; Liu, R.; Ji, S. Enhanced Multiferroic Properties of YMnO3 Ceramics Fabricated by Spark Plasma Sintering along with Low-Temperature Solid-State Reaction. Materials 2017, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Mao, Y. Recent Developments on Molten Salt Synthesis of Inorganic Nanomaterials: A Review. J. Phys. Chem. C 2021, 125, 6508–6533. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mao, Y. A Review on Molten Salt Synthesis of Metal Oxide Nanomaterials: Status, Opportunity, and Challenge. Prog. Mater. Sci. 2021, 117, 100734. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Wang, Z.; Liu, H.; Li, Y.; Wang, X.; Wei, H.; Cheng, G.J. Controllable Near-Infrared Reflectivity and Infrared Emissivity with Substitutional Iron-Doped Orthorhombic YMnO3 Coatings. Sol. Energy 2020, 206, 778–786. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Wang, Z.; Wang, X.; Liu, H.; Cheng, G.J. Molten Salt Synthesis of YMnO3 Powder with High Near-Infrared Reflectivity. Mater. Lett. 2018, 229, 171–173. [Google Scholar] [CrossRef]
- McBean, C.L.; Lewis, C.S.; Tiano, A.L.; Simonson, J.W.; Han, M.-G.; Gannon, W.J.; Yue, S.; Patete, J.M.; Corrao, A.A.; Santulli, A.C. A Generalizable Multigram Synthesis and Mechanistic Investigation of YMnO3 Nanoplates. Ind. Eng. Chem. Res. 2017, 56, 5573–5585. [Google Scholar] [CrossRef]
- Jian, G.; Xu, Y.; Lai, L.-C.; Wang, C.; Zachariah, M.R. Mn3O4 Hollow Spheres for Lithium-Ion Batteries with High Rate and Capacity. J. Mater. Chem. A Mater. 2014, 2, 4627–4632. [Google Scholar] [CrossRef]
- Broström, M.; Enestam, S.; Backman, R.; Mäkelä, K. Condensation in the KCl–NaCl System. Fuel Process. Technol. 2013, 105, 142–148. [Google Scholar] [CrossRef]
- Gizowska, M.; Piątek, M.; Perkowski, K.; Konopka, G.; Witosławska, I. Fabrication of Nanoyttria by Method of Solution Combustion Synthesis. Nanomaterials 2020, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Mishra, A. Structural and Electrical Properties of YMnO3 Manganites: Influence of Cr Ion Doping. J. Solid State Chem. 2021, 301, 122364. [Google Scholar] [CrossRef]
- Karoblis, D.; Zarkov, A.; Garskaite, E.; Mazeika, K.; Baltrunas, D.; Niaura, G.; Beganskiene, A.; Kareiva, A. Study of Gadolinium Substitution Effects in Hexagonal Yttrium Manganite YMnO3. Sci. Rep. 2021, 11, 2875. [Google Scholar] [CrossRef]
- Rao, G.V.S.; Rao, C.N.R. Infrared and Electronic Spectra of Rare Earth Perovskites. Ortho- Chromites, -Manganites and -Ferrites. Appl. Spectrosc. 1970, 24, 436–444. [Google Scholar] [CrossRef]
- Vishnuvardhan, T.K.; Kulkarni, V.R.; Basavaraja, C.; Raghavendra, S.C. Synthesis, Characterization and a.c. Conductivity of Polypyrrole/Y2O3 Composites. Bull. Mater. Sci. 2006, 29, 77–83. [Google Scholar] [CrossRef]
- Cherian, E.; Rajan, A.; Baskar, G. Synthesis of Manganese Dioxide Nanoparticles Using Co-Precipitation Method and Its Antimicrobial Activity. Int. J. Mod. Sci. Technol. 2016, 1, 17–22. [Google Scholar]
- Coşkun, M.; Polat, Ö.; Coşkun, F.M.; Durmuş, Z.; Çağlar, M.; Turut, A. Effect of Os Doping on Electrical Properties of YMnO3 Multiferroic Perovskite-Oxide Compounds. Mater. Sci. Semicond Process 2019, 91, 281–289. [Google Scholar] [CrossRef]
- Wan, F.; Li, L.; Bai, X.; Wang, Y.; Gao, L.; Li, J.; Cao, C. Structure and Dielectric Relaxation Behaviors of Co-Doped YMnO3 Multiferroic Ceramics. J. Mater. Sci. Mater. Electron. 2022, 33, 17361–17371. [Google Scholar] [CrossRef]
- Banerjee, D.; Nesbitt, H.W. XPS study of reductive dissolution of birnessite by oxalate: Rates and mechanistic aspects of dissolution and redox processes. Geochim. Et Cosmochim. Acta 1999, 51, 3025–3038. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Joshi, Z.; Dhruv, D.; Rathod, K.N.; Boricha, H.; Gadani, K.; Pandya, D.D.; Joshi, A.D.; Solanki, P.S.; Shah, N.A. Low Field Magnetoelectric Studies on Sol–Gel Grown Nanostructured YMnO3 Manganites. Prog. Solid State Chem. 2018, 49, 23–36. [Google Scholar] [CrossRef]
- Chanu, L.P.; Phanjoubam, S. Study on the Structural and Electrical Properties of YMnO3 Co-Substituted with Transition Metal Ions at Mn-Site and Their Conduction Mechanism. J. Mater. Sci. Mater. Electron. 2022, 33, 6107–6120. [Google Scholar] [CrossRef]









| Synthesis Conditions | a, Å | b, Å | c, Å | V, Å3 | |
|---|---|---|---|---|---|
| Temperature, °C | Molar Ratio between Precursors and Chlorides | ||||
| 900 | 1:2 | 6.1490 (6) | 6.1490 (6) | 11.4065 (7) | 373.51 (0) |
| 1100 | 1:2 | 6.1359 (4) | 6.1359 (4) | 11.3902 (5) | 371.38 (6) |
| 1000 | 1:10 | 6.1432 (5) | 6.1432 (5) | 11.4039 (0) | 372.71 (8) |
| 1100 | 1:10 | 6.1376 (1) | 6.1376 (1) | 11.3907 (3) | 371.60 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karoblis, D.; Zarkov, A.; Murauskas, T.; Kareiva, A. Molten Salt Synthesis of Micro-Sized Hexagonally Shaped REMnO3 (RE = Y, Er, Tm, Yb). Inorganics 2023, 11, 178. https://doi.org/10.3390/inorganics11050178
Karoblis D, Zarkov A, Murauskas T, Kareiva A. Molten Salt Synthesis of Micro-Sized Hexagonally Shaped REMnO3 (RE = Y, Er, Tm, Yb). Inorganics. 2023; 11(5):178. https://doi.org/10.3390/inorganics11050178
Chicago/Turabian StyleKaroblis, Dovydas, Aleksej Zarkov, Tomas Murauskas, and Aivaras Kareiva. 2023. "Molten Salt Synthesis of Micro-Sized Hexagonally Shaped REMnO3 (RE = Y, Er, Tm, Yb)" Inorganics 11, no. 5: 178. https://doi.org/10.3390/inorganics11050178
APA StyleKaroblis, D., Zarkov, A., Murauskas, T., & Kareiva, A. (2023). Molten Salt Synthesis of Micro-Sized Hexagonally Shaped REMnO3 (RE = Y, Er, Tm, Yb). Inorganics, 11(5), 178. https://doi.org/10.3390/inorganics11050178