Gadolinium(III)-DOTA Complex Functionalized with BODIPY as a Potential Bimodal Contrast Agent for MRI and Optical Imaging
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Gd-DOTA-BODIPY Derivative





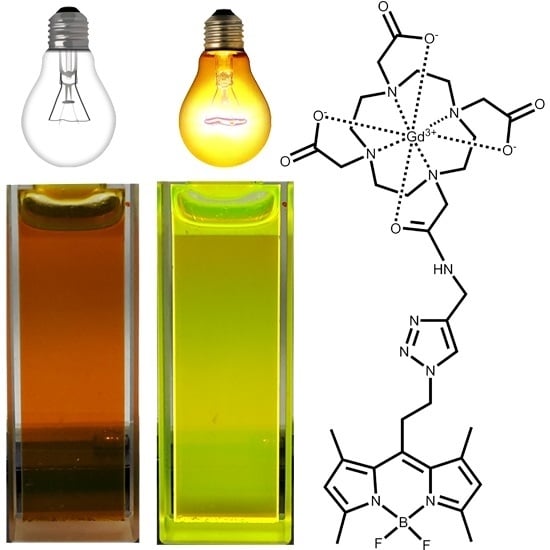
2.2. Photophysical Properties of the Gd-DOTA-BODIPY

2.3. Relaxivity of Gd-DOTA-BODIPY
3. Experimental Section
3.1. Materials, Reagents, and Solvents
3.2. Characterization
3.3. Synthetic Procedures
3.3.1. Synthesis of Product (1) (Figure 5)

3.3.2. Synthesis of BODIPY-(CH2)2-Phtalimide (2) (Figure 6)

3.3.3. Synthesis of BODIPY-(CH2)2-Amine (3) (Figure 7)

3.3.4. Synthesis of DO3A-tBu (4) (Figure 8)

3.3.5. Synthesis of {4,10-Bis-Tert-Butoxycarbonylmethyl-7-[(2-Propynylcarbamoyl)-Methyl]-1,4,7,10-Tetraaza-Cyclododec-1-yl}-Acetic Acid Tert-Butyl Ester (5) (Figure 9)

3.3.6. Synthesis of {4,10-Bis-Carboxymethyl-7-[(2-Propynylcarbamoyl)-Methyl]-1,4,7,10-Tetraaza-Cyclododec-1-yl}-Acetic Acid (6) (Figure 10)

3.3.7. Synthesis of Lanthanide(III) {4,10-Bis-Carboxymethyl-7-[(2-Propynylcarbamoyl)-Methyl]-1,4,7,10-Tetraaza-Cyclododec-1-yl}-Acetic Acid (Figure 11)

3.3.8. General Procedure Flow

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hermann, P.; Kotek, J.; Kubícek, V.; Lukes, I. Gadolinium(III) complexes as MRI contrast agents: Ligand design and properties of the complexes. Dalton Trans. 2008, 9226, 3027–3047. [Google Scholar] [CrossRef] [PubMed]
- Pierre, V.C.; Allen, M.J.; Caravan, P. Contrast agents for MRI: 30+ years and where are we going? J. Biol. Inorg. Chem. 2014, 19, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.A.; Wickline, S.A. Contrast agents for MRI. Basic Res. Cardiol. 2008, 103, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Tóth, É.; Helm, L.; Merbach, A. Contrast Agents I. In Topics in Current Chemistry; Krause, W., Ed.; Springer Berlin Heidelberg: Berlin, Germany, 2002; Volume 221, pp. 61–101. [Google Scholar]
- Debroye, E.; Parac-Vogt, T.N. Towards polymetallic lanthanide complexes as dual contrast agents for magnetic resonance and optical imaging. Chem. Soc. Rev. 2014, 43, 8178–8192. [Google Scholar] [CrossRef] [PubMed]
- Cacheris, W.P.; Quay, S.C.; Rocklage, S.M. The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn. Reson. Imaging 1990, 8, 467–481. [Google Scholar] [CrossRef]
- Shellock, F.G.; Kanal, E. Safety of magnetic resonance imaging contrast agents. J. Magn. Reson. Imaging 1999, 10, 477–484. [Google Scholar] [CrossRef]
- Bartolini, M.E.; Pekar, J.; Chettle, D.R.; McNeill, F.; Scott, A.; Sykes, J.; Prato, F.S.; Moran, G.R. An investigation of the toxicity of gadolinium based MRI contrast agents using neutron activation analysis. Magn. Reson. Imaging 2003, 21, 541–544. [Google Scholar] [CrossRef]
- Accardo, A.; Tesauro, D.; Aloj, L.; Pedone, C.; Morelli, G. Supramolecular aggregates containing lipophilic Gd(III) complexes as contrast agents in MRI. Coord. Chem. Rev. 2009, 253, 2193–2213. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Villaraza, A.J.L.; Bumb, A.; Brechbiel, M.W. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: The interplay between size, function, and pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. [Google Scholar] [CrossRef] [PubMed]
- Sherry, A.D.; Brown, R.D.; Geraldes, C.F.G.C.; Koenig, S.H.; Kuan, K.-T.; Spiller, M. Synthesis and characterization of the gadolinium(3+) complex of DOTA-propylamide: A model DOTA-protein conjugate. Inorg. Chem. 1989, 28, 620–622. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Davis, J.J. Multimodality and nanoparticles in medical imaging. Dalton Trans. 2011, 40, 6087–6103. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, G.; Eden, H.S.; Ai, H.; Chen, X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.P.; Wang, T.D. Exogenous molecular probes for targeted imaging in cancer: Focus on multi-modal imaging. Cancers (Basel) 2010, 2, 1251–1287. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Vande Velde, G.; Ketkar-Atre, A.; Himmelreich, U.; de Cuyper, M. Magnetoliposomes as magnetic resonance imaging contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2002, 3, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Ruloff, R.; Burai, L.; Helm, L.; Merbach, A.E. Rigid M(II)L2Gd2(III) (M = Fe, Ru) complexes of a terpyridine-based heteroditopic chelate: A class of candidates for MRI contrast agents. J. Am. Chem. Soc. 2005, 127, 5147–5157. [Google Scholar] [CrossRef] [PubMed]
- Livramento, J.B.; Sour, A.; Borel, A.; Merbach, A.E.; Tóth, É. A starburst-shaped heterometallic compound incorporating six densely packed Gd3+ Ions. Chem. Eur. J. 2006, 12, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Livramento, J.B.; Weidensteiner, C.; Prata, M.I.M.; Allegrini, P.R.; Geraldes, C.F.G.C.; Helm, L.; Kneuer, R.; Merbach, A.E.; Santos, A.C.; Schmidt, P.; et al. First in vivo MRI assessment of a self-assembled metallostar compound endowed with a remarkable high field relaxivity. Contrast Media Mol. Imaging 2006, 1, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Parac-Vogt, T.N.; Vander Elst, L.; Kimpe, K.; Laurent, S.; Burtéa, C.; Chen, F.; van Deun, R.; Ni, Y.; Muller, R.N.; Binnemans, K. Pharmacokinetic and in vivo evaluation of a self-assembled gadolinium(III)-iron(II) contrast agent with high relaxivity. Contrast Media Mol. Imaging 2006, 1, 267–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, J.; Gameiro, C.; Humblet, V.; Mohapatra, P.K.; Jacques, V.; Desreux, J.F. Auto-assembling of ditopic macrocyclic lanthanide chelates with transition-metal ions. Rigid multimetallic high relaxivity contrast agents for magnetic resonance imaging. Inorg. Chem. 2006, 45, 5092–5102. [Google Scholar] [CrossRef] [PubMed]
- Dehaen, G.; Verwilst, P.; Eliseeva, S.V.; Laurent, S.; Vander Elst, L.; Muller, R.N.; de Borggraeve, W.M.; Binnemans, K.; Parac-Vogt, T.N. A heterobimetallic ruthenium-gadolinium complex as a potential agent for bimodal imaging. Inorg. Chem. 2011, 50, 10005–10014. [Google Scholar] [CrossRef] [PubMed]
- Dehaen, G.; Eliseeva, S.V.; Kimpe, K.; Laurent, S.; Vander Elst, L.; Muller, R.N.; Dehaen, W.; Binnemans, K.; Parac-Vogt, T.N.; Vanderelst, L.; et al. A self-assembled complex with a titanium(IV) catecholate core as a potential bimodal contrast agent. Chem. Eur. J. 2012, 18, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Verwilst, P.; Eliseeva, S.V.; Vander Elst, L.; Burtea, C.; Laurent, S.; Petoud, S.; Muller, R.N.; Parac-Vogt, T.N.; de Borggraeve, W.M. A tripodal ruthenium-gadolinium metallostar as a potential αvβ3 integrin specific bimodal imaging contrast agent. Inorg. Chem. 2012, 51, 6405–6411. [Google Scholar] [CrossRef] [PubMed]
- Dehaen, G.; Eliseeva, S.V.; Verwilst, P.; Laurent, S.; Vander Elst, L.; Muller, R.N.; de Borggraeve, W.M.; Binnemans, K.; Parac-Vogt, T.N. Tetranuclear d-f metallostars: Synthesis, relaxometric, and luminescent properties. Inorg. Chem. 2012, 51, 8775–8783. [Google Scholar] [CrossRef] [PubMed]
- Debroye, E.; Ceulemans, M.; Vander Elst, L.; Laurent, S.; Muller, R.N.; Parac-Vogt, T.N. Controlled synthesis of a novel heteropolymetallic complex with selectively incorporated lanthanide(III) ions. Inorg. Chem. 2014, 53, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Kuil, J.; Buckle, T.; Oldenburg, J.; Yuan, H.; Borowsky, A.D.; Josephson, L.; van Leeuwen, F.W.B. Hybrid peptide dendrimers for imaging of chemokine receptor 4 (CXCR4) expression. Mol. Pharm. 2011, 8, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Musonda, C.C.; Taylor, D.; Lehman, J.; Gut, J.; Rosenthal, P.J.; Chibale, K. Application of multi-component reactions to antimalarial drug discovery. Part 1: Parallel synthesis and antiplasmodial activity of new 4-aminoquinoline Ugi adducts. Bioorganic Med. Chem. Lett. 2004, 14, 3901–3905. [Google Scholar] [CrossRef] [PubMed]
- Rivas, C.; Stasiuk, G.J.; Sae-Heng, M.; Long, N.J. Towards understanding the design of dual-modal MR/fluorescent probes to sense zinc ions. Dalton Trans. 2015, 44, 4976–4985. [Google Scholar] [CrossRef] [PubMed]
- Hüber, M.M.; Staubli, A.B.; Kustedjo, K.; Gray, M.H.B.; Shih, J.; Fraser, S.E.; Jacobs, R.E.; Meade, T.J. Fluorescently detectable magnetic resonance imaging agents. Bioconjug. Chem. 1998, 9, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.A.K.; Pfeuffer, J.; Mishra, R.; Engelmann, J.; Mishra, A.A.K.; Ugurbil, K.; Logothetis, N.K. A new class of Gd-based DO3A-ethylamine-derived targeted contrast agents for MR and optical imaging. Bioconjug. Chem. 2006, 17, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Imm, S.; Bähn, S.; Neubert, L.; Neumann, H.; Beller, M. Efficient copper(II)-catalyzed transamidation of non-activated primary carboxamides and ureas with amines. Angew. Chem. Int. Ed. 2012, 51, 3905–3909. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Bhuniya, S.; Kang, J.; Yeom, A.; Hong, K.S.; Kim, J.S. Cu2+-responsive bimodal (optical/MRI) contrast agent for cellular imaging. Org. Lett. 2013, 15, 4702–4705. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.-C.; Fas, S.C.; Stohlmeyer, M.M.; Wandless, T.J.; Cimprich, K.A. A cell-permeable, activity-based probe for protein and lipid kinases. J. Biol. Chem. 2005, 280, 29053–29059. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Panagabko, C.; Atkinson, J. Synthesis and characterization of BODIPY-α-tocopherol: A fluorescent form of vitamin E. J. Org. Chem. 2010, 75, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, I.; Amat-Guerri, F.; Liras, M.; Costela, A.; Infantes, L.; Sastre, R.; López Arbeloa, F.; Bañuelos Prieto, J.; López Arbeloa, Í. Structural changes in the BODIPY dye PM567 enhancing the laser action in liquid and solid media. Adv. Funct. Mater. 2007, 17, 3088–3098. [Google Scholar] [CrossRef]
- Duran-Sampedro, G.; Agarrabeitia, A.R.; Garcia-Moreno, I.; Costela, A.; Bañuelos, J.; Arbeloa, T.; López Arbeloa, I.; Chiara, J.L.; Ortiz, M.J. Chlorinated BODIPYs: Surprisingly efficient and highly photostable laser dyes. Eur. J. Org. Chem. 2012, 2012, 6335–6350. [Google Scholar] [CrossRef]
- Lakshmi, V.; Rajeswara Rao, M.; Ravikanth, M. Halogenated boron-dipyrromethenes: Synthesis, properties and applications. Org. Biomol. Chem. 2015, 13, 2501–2517. [Google Scholar] [CrossRef] [PubMed]
- Bessette, A.; Hanan, G.S. Design, synthesis and photophysical studies of dipyrromethene-based materials: Insights into their applications in organic photovoltaic devices. Chem. Soc. Rev. 2014, 43, 3342. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Gayathri, T. Evolution of BODIPY dyes as potential sensitizers for dye-sensitized solar cells. Eur. J. Org. Chem. 2014, 2014, 4689–4707. [Google Scholar] [CrossRef]
- González-Béjar, M.; Liras, M.; Francés-Soriano, L.; Voliani, V.; Herranz-Pérez, V.; Duran-Moreno, M.; Garcia-Verdugo, J.M.; Alarcon, E.I.; Scaiano, J.C.; Pérez-Prieto, J. NIR excitation of upconversion nanohybrids containing a surface grafted bodipy induces oxygen-mediated cancer cell death. J. Mater. Chem. B 2014, 2, 4554. [Google Scholar] [CrossRef]
- Lim, S.H.; Thivierge, C.; Nowak-Sliwinska, P.; Han, J.; van den Bergh, H.; Wagnières, G.; Burgess, K.; Lee, H.B. In vitro and in vivo photocytotoxicity of boron dipyrromethene derivatives for photodynamic therapy. J. Med. Chem. 2010, 53, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, C.; Goze, C.; Rousselin, Y.; Denat, F. First bodipy–DOTA derivatives as probes for bimodal imaging. Chem. Commun. 2010, 46, 8267. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, C.; Moreau, M.; Lhenry, D.; Goze, C.; Boschetti, F.; Rousselin, Y.; Brunotte, F.; Denat, F. DOTAGA-anhydride: A valuable building block for the preparation of DOTA-like chelating agents. Chem. Eur. J. 2012, 18, 7834–7841. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, S.; Hokamura, K.; Ogawa, M.; Takehara, Y.; Muramatsu, Y.; Yamane, T.; Hirabayashi, K.; Morimoto, Y.; Hagisawa, K.; Nakahara, K.; et al. A design strategy for small molecule-based targeted MRI contrast agents: Their application for detection of atherosclerotic plaques. Org. Biomol. Chem. 2014, 12, 8611–8618. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Hanaoka, K.; Muramatsu, Y.; Tamura, K.; Adachi, Y.; Miyashita, Y.; Hirata, Y.; Nagano, T. Method for enhancing cell penetration of Gd3+-based MRI contrast agents by conjugation with hydrophobic fluorescent dyes. Bioconjug. Chem. 2011, 22, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Duheron, V.; Moreau, M.; Collin, B.; Sali, W.; Bernhard, C.; Goze, C.; Gautier, T.; Pais de Barros, J.-P.; Deckert, V.; Brunotte, F.; et al. Dual labeling of lipopolysaccharides for SPECT-CT imaging and fluorescence microscopy. ACS Chem. Biol. 2014, 9, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Lhenry, D.; Larrouy, M.; Bernhard, C.; Goncalves, V.; Raguin, O.; Provent, P.; Moreau, M.; Collin, B.; Oudot, A.; Vrigneaud, J.-M.; et al. BODIPY: A highly versatile platform for the design of bimodal imaging probes. Chemistry 2015, 21, 13091–13099. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, J.A.; Keliher, E.J.; Wan, D.; Hilderbrand, S.A.; Weissleder, R.; Mazitschek, R. Synthesis of [18F]BODIPY: Bifunctional reporter for hybrid optical/positron emission tomography imaging. Angew. Chem. Int. Ed. Engl. 2012, 51, 4603–4606. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, D.; Zhang, Z.; Surya Prakash, G.K.; Conti, P.S.; Li, Z. Efficient synthesis of fluorescent-PET probes based on [18F]BODIPY dye. Chem. Commun. 2014, 50, 7371. [Google Scholar] [CrossRef] [PubMed]
- Nuyts, K.; Ceulemans, M.; Parac-Vogt, T.N.; Bultynck, G.; de Borggraeve, W.M. Facile azide formation via diazotransfer reaction in a copper tube flow reactor. Tetrahedron Lett. 2015, 56, 1687–1690. [Google Scholar] [CrossRef]
- Fischer, N.; Goddard-Borger, E.D.; Greiner, R.; Klapötke, T.M.; Skelton, B.W.; Stierstorfer, J. Sensitivities of some imidazole-1-sulfonyl azide salts. J. Org. Chem. 2012, 77, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.M.; Sewell, A.L.; Pedersen, R.H.; Long, D.-L.; Gadegaard, N.; Marquez, R. Tunable BODIPY derivatives amenable to “click” and peptide chemistry. Tetrahedron 2013, 69, 8527–8533. [Google Scholar] [CrossRef]
- Viguier, R.F.H.; Hulme, A.N. A sensitized europium complex generated by micromolar concentrations of copper(I): Toward the detection of copper(I) in biology. J. Am. Chem. Soc. 2006, 128, 11370–11371. [Google Scholar] [CrossRef] [PubMed]
- Verwilst, P.; Eliseeva, S.V.; Carron, S.; Vander Elst, L.; Burtea, C.; Dehaen, G.; Laurent, S.; Binnemans, K.; Muller, R.N.; Parac-Vogt, T.N.; et al. A modular approach towards the synthesis of target-specific MRI contrast agents. Eur. J. Inorg. Chem. 2011, 2011, 3577–3585. [Google Scholar] [CrossRef]
- Hessel, V.; Kralisch, D.; Kockmann, N.; Noël, T.; Wang, Q. Novel process windows for enabling, accelerating, and uplifting flow chemistry. ChemSusChem 2013, 6, 746–789. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Pedersen, D.S. Azide- and alkyne-derivatised α-amino acids. Eur. J. Org. Chem. 2012, 2012, 4267–4281. [Google Scholar] [CrossRef]
- Goddard-Borger, E.D.; Stick, R.V. An efficient, inexpensive, and shelf-stable diazotransfer reagent: Imidazole-1-sulfonyl azide hydrochloride. Org. Lett. 2007, 9, 3797–3800. [Google Scholar] [CrossRef] [PubMed]
- Duimstra, J.A.; Femia, F.J.; Meade, T.J. A gadolinium chelate for detection of β-glucuronidase: A self-immolative approach. J. Am. Chem. Soc. 2005, 127, 12847–12855. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Verbelen, B.; Tonnelé, C.; Beljonne, D.; Lazzaroni, R.; Leen, V.; Dehaen, W.; Boens, N. UV-VIS spectroscopy of the coupling products of the palladium-catalyzed C–H arylation of the BODIPY core. Photochem. Photobiol. Sci. 2013, 12, 835–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef] [PubMed]
- Niu, S. Advanced Water Soluble BODIPY Dyes: Synthesis and Application. Ph.D. Thesis, Université de Strasbourg, Strasbourg, France, July 2011. [Google Scholar]
- Olmsted, J. Calorimetric determinations of absolute fluorescence quantum yields. J. Phys. Chem. 1979, 83, 2581–2584. [Google Scholar] [CrossRef]
- Solomon, I. Relaxation processes in a system of two spins. Phys. Rev. 1955, 99, 559–565. [Google Scholar] [CrossRef]
- Bloembergen, N. Proton relaxation times in paramagnetic solutions. J. Chem. Phys. 1957, 27, 572. [Google Scholar] [CrossRef]
- Laurent, S.; Elst, L.V.; Muller, R.N. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol. Imaging 2006, 1, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H. Spectrophotometric determination of zirconium, uranium, thorium and rare earths with arsenazo III after extractions with thenoyltrifluoroacetone and tri-n-octylamine. Talanta 1972, 19, 473–478. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceulemans, M.; Nuyts, K.; De Borggraeve, W.M.; Parac-Vogt, T.N. Gadolinium(III)-DOTA Complex Functionalized with BODIPY as a Potential Bimodal Contrast Agent for MRI and Optical Imaging. Inorganics 2015, 3, 516-533. https://doi.org/10.3390/inorganics3040516
Ceulemans M, Nuyts K, De Borggraeve WM, Parac-Vogt TN. Gadolinium(III)-DOTA Complex Functionalized with BODIPY as a Potential Bimodal Contrast Agent for MRI and Optical Imaging. Inorganics. 2015; 3(4):516-533. https://doi.org/10.3390/inorganics3040516
Chicago/Turabian StyleCeulemans, Matthias, Koen Nuyts, Wim M. De Borggraeve, and Tatjana N. Parac-Vogt. 2015. "Gadolinium(III)-DOTA Complex Functionalized with BODIPY as a Potential Bimodal Contrast Agent for MRI and Optical Imaging" Inorganics 3, no. 4: 516-533. https://doi.org/10.3390/inorganics3040516
APA StyleCeulemans, M., Nuyts, K., De Borggraeve, W. M., & Parac-Vogt, T. N. (2015). Gadolinium(III)-DOTA Complex Functionalized with BODIPY as a Potential Bimodal Contrast Agent for MRI and Optical Imaging. Inorganics, 3(4), 516-533. https://doi.org/10.3390/inorganics3040516