Si–H Bond Activation of a Primary Silane with a Pt(0) Complex: Synthesis and Structures of Mononuclear (Hydrido)(dihydrosilyl) Platinum(II) Complexes
Abstract
:1. Introduction
2. Results
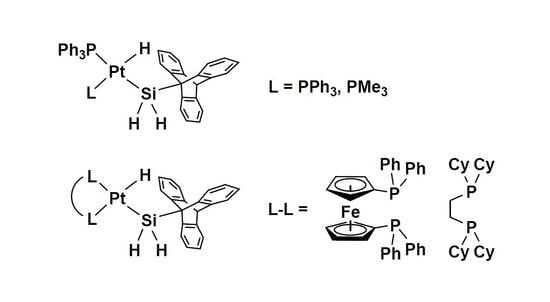
2.1. Synthesis and Characterization of [cis-PtH(SiH2Trip)(PPh3)2] (2)
2.2. Ligand Exchange Reactions of 2 with Free Phosphine Ligands
3. Materials and Methods
3.1. General Procedures
3.1.1. [cis-PtH(SiH2Trip)(PPh3)2] (2)
3.1.2. [PtH(SiH2Trip)(dppf)] (3)
3.1.3. [PtH(SiH2Trip)(dcpe)] (4)
3.1.4. [PtH(SiH2Trip)(PMe3)(PPh3)] (5)
3.2. X-ray Crystallographic Studies of 2, 3, and 5
3.2.1. [cis-PtH(SiH2Trip)(PPh3)2] (2)
3.2.2. [PtH(SiH2Trip)(dppf)] (3)
3.2.3. [PtH(SiH2Trip)(PMe3)(PPh3)] (5)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Corey, J.Y. Reactions of hydrosilanes with transition metal complexes. Chem. Rev. 2016, 116, 11291–11435. [Google Scholar] [CrossRef] [PubMed]
- Marciniec, B. Comprehensive Handbook on Hydrosilylation; Pergamon Press: Oxford, UK, 1992; ISBN 0-08-040272-0. [Google Scholar]
- Ojima, I.; Li, Z.; Zhu, J. Recent Advances in Hydrosilylation and Related Reactions. In The Chemistry of Organic Silicon Compounds; Patai, S., Rappoport, Z., Eds.; John Wiley and Sons: New York, NY, USA, 1998; pp. 1687–1792. [Google Scholar]
- Marciniec, B.; Maciejewski, H.; Pietraszuk, C.; Pawluc, P. Hydrosilylation: A Comprehensive Review on Recent Advances; Marciniec, B., Ed.; Advances in Silicon Science; Springer: Berlin, Germany, 2009; Volume 1, ISBN 978-1-4020-8172-9. [Google Scholar]
- Chalk, A.J.; Harrod, J.F. Homogeneous catalysis. II. The mechanism of the hydrosilation of olefins catalyzed by group VIII metal complexes. J. Am. Chem. Soc. 1965, 87, 16–21. [Google Scholar] [CrossRef]
- Ozawa, F. The chemistry of organo(silyl)platinum(II) complexes relevant to catalysis. J. Organomet. Chem. 2000, 611, 332–342. [Google Scholar] [CrossRef]
- Sharma, H.; Pannell, K.H. Activation of the Si–Si bond by transition metal complexes. Chem. Rev. 1995, 95, 1351–1374. [Google Scholar] [CrossRef]
- Suginome, M.; Ito, Y. Transition-metal-catalyzed additions of silicon−silicon and silicon−heteroatom bonds to unsaturated organic molecules. Chem. Rev. 2000, 100, 3221–3256. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I.; Inaba, S.-I.; Kogure, T.; Nagai, Y. The action of tris(triphenylphosphine)chlororhodium on polyhydromonosilanes. J. Organomet. Chem. 1973, 55, C7–C8. [Google Scholar] [CrossRef]
- Chang, L.S.; Johnson, M.P.; Fink, J. Polysilyl complexes of platinum—Synthesis and thermochemistry. Organometallics 1989, 8, 1369–1371. [Google Scholar] [CrossRef]
- Harrod, J.F.; Mu, Y.; Samuel, E. Catalytic dehydrocoupling: A general method for the formation of element-element bonds. Polyhedron 1991, 10, 1239–1245. [Google Scholar] [CrossRef]
- Woo, H.G.; Walzer, J.F.; Tilley, T.D. A σ-bond metathesis mechanism for dehydropolymerization of silanes to polysilanes by d0 metal catalysts. J. Am. Chem. Soc. 1992, 114, 7047–7055. [Google Scholar] [CrossRef]
- Grimmond, B.J.; Corey, J.Y. Amino-functionalized zirconocene (C5H4CH(Me)NMe2)2ZrCl2, a catalyst for the dehydropolymerization of PhSiH3: Probing of peripheral substituent effects on catalyst dehydropolymerization activity. Inorg. Chim. Acta 2002, 330, 89–94. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Park, J.-I.; Lee, S.-C.; Osakada, K.; Tanabe, M.; Choi, J.-C.; Koizumi, T.; Yamamoto, T. Cis and trans isomers of Pt(SiHAr2)2(PR3)2 (R = Me, Et) in the solid state and in solutions. Organometallics 1999, 18, 1349–1352. [Google Scholar] [CrossRef]
- Braddock-Wilking, J.; Corey, J.Y.; Trankler, K.A.; Xu, H.; French, L.M.; Praingam, N.; White, C.; Rath, N.P. Spectroscopic and reactivity studies of platinum−silicon monomers and dimers. Organometallics 2006, 25, 2859–2871. [Google Scholar] [CrossRef]
- Arii, H.; Takahashi, M.; Noda, A.; Nanjo, M.; Mochida, K. Spectroscopic and structural studies of thermally unstable intermediates generated in the reaction of [Pt(PPh3)2(η2-C2H4)] with dihydrodisilanes. Organometallics 2008, 27, 1929–1935. [Google Scholar] [CrossRef]
- Ahrens, T.; Braun, T.; Braun, B. Activation of 1,2-dihydrodisilanes at platinum(0) phosphine complexes: Syntheses and structures of bissilyl platinum(II) complexes. Z. Anorg. Allg. Chem. 2014, 640, 93–99. [Google Scholar] [CrossRef]
- Li, Y.-H.; Huang, Z.-F.; Li, X.-A.; Lai, W.-Y.; Wang, L.-H.; Ye, S.-H.; Cui, L.-F.; Wang, S. Synthesis and structural characterization of a novel bis(silyl) platinum(II) complex bearing SiH3 ligand. J. Orgnomet. Chem. 2014, 749, 246–250. [Google Scholar] [CrossRef]
- Auburn, M.; Ciriano, M.; Howard, J.A.K.; Murray, M.; Pugh, N.J.; Spencer, J.L.; Stone, F.G.A.; Woodward, P. Synthesis of bis-µ-diorganosilanediyl-af-dihydridobis(triorganophosphine)diplatinum complexes: Crystal and molecular structure of [{PtH(μ-SiMe2)[P(C6H11)3]}2]. J. Chem. Soc. Dalton Trans. 1980, 659–666. [Google Scholar] [CrossRef]
- Zarate, E.A.; Tessier-Youngs, C.A.; Young, W.J. Synthesis and structural characterization of platinum–silicon dimers with unusually short cross-ring silicon–silicon interactions. J. Am. Chem. Soc. 1988, 110, 4068–4070. [Google Scholar] [CrossRef]
- Levchinsky, Y.; Rath, N.P.; Braddock-Wilking, J. Reaction of a symmetrical diplatinum complex containing bridging μ-η2-H−SiH(IMP) ligands (IMP = 2-isopropyl-6-methylphenyl) with PMe2Ph. Formation and characterization of {(PhMe2P)2Pt[μ-SiH(IMP)]}2. Organometallics 1999, 18, 2583–2586. [Google Scholar] [CrossRef]
- Sanow, L.M.; Chai, M.; McConnville, D.B.; Galat, K.J.; Simons, R.S.; Rinaldi, P.L.; Young, W.; Tessier, C.A. Platinum−silicon four-membered rings of two different structural types. Organometallics 2000, 19, 192–205. [Google Scholar] [CrossRef]
- Braddock-Wilking, J.; Levchinsky, Y.; Rath, N.P. Synthesis and characterization of diplatinum complexes containing bridging μ-η2-H−SiHAr ligands. X-ray crystal structure determination of {(Ph3P)Pt[μ-η2-H−SiHAr]}2 (Ar = 2,4,6-(CF3)3C6H2, C6Ph5). Organometallics 2000, 19, 5500–5510. [Google Scholar] [CrossRef]
- Osakada, K.; Tanabe, M.; Tanase, T. A triangular triplatinum complex with electron-releasing SiPh2 and PMe3 Ligands: [{Pt(μ-SiPh2)(PMe3)}3]. Angew. Chem. Int. Ed. 2000, 39, 4053–4054. [Google Scholar] [CrossRef]
- Braddock-Wilking, J.; Corey, J.Y.; Trankler, K.A.; Dill, K.M.; French, L.M.; Rath, N.P. Reaction of silafluorenes with (Ph3P)2Pt(η2-C2H4): Generation and characterization of Pt−Si monomers, dimers, and trimers. Organometallics 2004, 23, 4576–4584. [Google Scholar] [CrossRef]
- Braddock-Wilking, J.; Corey, J.Y.; French, L.M.; Choi, E.; Speedie, V.J.; Rutherford, M.F.; Yao, S.; Xu, H.; Rath, N.P. Si−H bond activation by (Ph3P)2Pt(η2-C2H4) in dihydrosilicon tricycles that also contain O and N heteroatoms. Organometallics 2006, 25, 3974–3988. [Google Scholar] [CrossRef]
- Tanabe, M.; Ito, D.; Osakada, K. Diplatinum complexes with bridging silyl ligands. Si−H bond activation of μ-silyl ligand leading to a new platinum complex with bridging silylene and silane ligands. Organometallics 2007, 26, 459–462. [Google Scholar] [CrossRef]
- Tanabe, M.; Ito, D.; Osakada, K. Ligand exchange of diplatinum complexes with bridging silyl ligands involving Si−H bond cleavage and formation. Organometallics 2008, 27, 2258–2267. [Google Scholar] [CrossRef]
- Tanabe, M.; Kamono, M.; Tanaka, K.; Osakada, K. Triangular triplatinum complex with four bridging Si ligands: Dynamic behavior of the molecule and catalysis. Organometallics 2017, 36, 1929–1935. [Google Scholar] [CrossRef]
- Mullica, D.F.; Sappenfield, E.L. Synthesis and X-ray structural investigation of cis-hydrido(triphenylsilyl)-1,4-butanediyl-bis-(dicyclohexylphosphine)platinum(II). Polyhedron 1991, 10, 867–872. [Google Scholar] [CrossRef]
- Latif, L.A.; Eaborn, C.; Pidcock, A.P. Square planar platinum(II) complexes. Crystal structures of cis-bis(triphenylphosphine)hydro (triphenylstannyl)platinum(II) and cis-bis(triphenylphosphine)-hydro(triphenylsilyl)platinum(II). J. Organomet. Chem. 1994, 474, 217–221. [Google Scholar] [CrossRef]
- Koizumi, T.; Osakada, K.; Yamamoto, T. Intermolecular transfer of triarylsilane from RhCl(H)(SiAr3)[P(i-Pr)3]2 to a platinum(0) complex, giving cis-PtH(SiAr3)(PEt3)2 (Ar = C6H5, C6H4F-p, C6H4Cl-p). Organometallics 1997, 16, 6014–6016. [Google Scholar] [CrossRef]
- Chan, D.; Duckett, S.B.; Heath, S.L.; Khazal, I.G.; Perutz, R.N.; Sabo-Etienne, S.; Timmins, P.L. Platinum bis(tricyclohexylphosphine) silyl hydride complexes. Organometallics 2004, 23, 5744–5756. [Google Scholar] [CrossRef]
- Arii, H.; Takahashi, M.; Nanjo, M.; Mochida, K. Syntheses of mono- and dinuclear silylplatinum complexes bearing a diphosphino ligand via stepwise bond activation of unsymmetric disilanes. Dalton Trans. 2010, 39, 6434–6440. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.S.; Sanow, L.M.; Galat, K.J.; Tessier, C.A.; Young, W.J. Synthesis and Structural Characterization of the Mono(silyl)platinum(II) Complex cis-(2,6-Mes2C6H3(H)2Si)Pt(H)(PPr3)2. Organometallics 2000, 19, 3994–3996. [Google Scholar] [CrossRef]
- Wang, S.; Yu, X.-F.; Li, N.; Yang, T.; Lai, W.-Y.; Mi, B.-X.; Li, J.-F.; Li, Y.-H.; Wang, L.-H.; Huang, W. Synthesis and structural studies of a rare bis(phosphine) (hydrido) (silyl) platinum(II) complex containing a Si–Si single bond. J. Orgnomet. Chem. 2015, 776, 113–116. [Google Scholar] [CrossRef]
- Nakata, N.; Fukazawa, S.; Ishii, A. Synthesis and crystal structures of the first stable mononuclear dihydrogermyl(hydrido) platinum(II) complexes. Organometallics 2009, 28, 534–538. [Google Scholar] [CrossRef]
- Nakata, N.; Fukazawa, S.; Kato, N.; Ishii, A. Palladium(II) hydrido complexes having a primary silyl or germyl ligand: Synthesis, crystal structures, and dynamic behavior. Organometallics 2011, 30, 4490–4493. [Google Scholar] [CrossRef]
- Nakata, T.; Sekizawa, N.; Ishii, A. Cationic dinuclear platinum and palladium complexes with bridging hydrogermylene and hydrido ligands. Chem. Commun. 2015, 51, 10111–10114. [Google Scholar] [CrossRef] [PubMed]
- Kalläne, S.; Laubenstein, R.; Braun, T.; Dietrich, M. Activation of Si–Si and Si–H bonds at a platinum bis(diphenylphosphanyl)ferrocene (dppf) complex: Key steps for the catalytic hydrogenolysis of disilanes. Eur. J. Inorg. Chem. 2016, 530–537. [Google Scholar] [CrossRef]
- Boyle, R.C.; Mague, J.T.; Fink, M.J. The first stable mononuclear silyl palladium hydrides. J. Am. Chem. Soc. 2003, 125, 3228–3229. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.C.; Pool, D.; Jacobsen, H.; Fink, M.J. Dynamic processes in silyl palladium complexes: Evidence for intermediate Si−H and Si−Si σ-complexes. J. Am. Chem. Soc. 2006, 128, 9054–9055. [Google Scholar] [CrossRef] [PubMed]
- Feducia, J.A.; Campbell, A.N.; Doherty, M.Q.; Gagné, M.R. Modular catalysts for diene cycloisomerization: Rapid and enantioselective variants for bicyclopropane synthesis. J. Am. Chem. Soc. 2006, 128, 13290–13297. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.N.; Gagné, M.R. Room-temperature β-H elimination in (P2P)Pt(OR) cations: Convenient synthesis of a platinum hydride. Organometallics 2007, 26, 2788–2790. [Google Scholar] [CrossRef]
- Pan, B.; Xu, Z.; Bezpalko, M.W.; Foxman, B.M.; Thomas, C.M. N-Heterocyclic phosphenium ligands as sterically and electronically-tunable isolobal analogues of nitrosyls. Inorg. Chem. 2012, 51, 4170–4179. [Google Scholar] [CrossRef] [PubMed]
- Frey, U.; Helm, L.; Merbach, A.E.; Romeo, R. High-pressure NMR kinetics. Part 41. Dissociative substitution in four-coordinate planar platinum(II) complexes as evidenced by variable-pressure high-resolution proton NMR magnetization transfer experiments. J. Am. Chem. Soc. 1989, 111, 8161–8165. [Google Scholar] [CrossRef]
- Romeo, R.; Grassi, A.; Scolaro, L.M. Factors affecting reaction pathways in nucleophilic substitution reactions on platinum(II) complexes: A comparative kinetic and theoretical study. Inorg. Chem. 1992, 31, 4383–4390. [Google Scholar] [CrossRef]
- Brynda, M.; Bernardinelli, G.; Dutan, C.; Geoffroy, M. Kinetic stabilization of primary hydrides of main group elements. The synthesis of an air-stable, crystalline arsine and silane. Inorg. Chem. 2003, 42, 6586–6588. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.D.; Jauhal, G.S. Chemistry of low-valent complexes. II. Cyclic azo derivatives of platinum. J. Am. Chem. Soc. 1968, 90, 1464–1467. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakata, N.; Kato, N.; Sekizawa, N.; Ishii, A. Si–H Bond Activation of a Primary Silane with a Pt(0) Complex: Synthesis and Structures of Mononuclear (Hydrido)(dihydrosilyl) Platinum(II) Complexes. Inorganics 2017, 5, 72. https://doi.org/10.3390/inorganics5040072
Nakata N, Kato N, Sekizawa N, Ishii A. Si–H Bond Activation of a Primary Silane with a Pt(0) Complex: Synthesis and Structures of Mononuclear (Hydrido)(dihydrosilyl) Platinum(II) Complexes. Inorganics. 2017; 5(4):72. https://doi.org/10.3390/inorganics5040072
Chicago/Turabian StyleNakata, Norio, Nanami Kato, Noriko Sekizawa, and Akihiko Ishii. 2017. "Si–H Bond Activation of a Primary Silane with a Pt(0) Complex: Synthesis and Structures of Mononuclear (Hydrido)(dihydrosilyl) Platinum(II) Complexes" Inorganics 5, no. 4: 72. https://doi.org/10.3390/inorganics5040072
APA StyleNakata, N., Kato, N., Sekizawa, N., & Ishii, A. (2017). Si–H Bond Activation of a Primary Silane with a Pt(0) Complex: Synthesis and Structures of Mononuclear (Hydrido)(dihydrosilyl) Platinum(II) Complexes. Inorganics, 5(4), 72. https://doi.org/10.3390/inorganics5040072