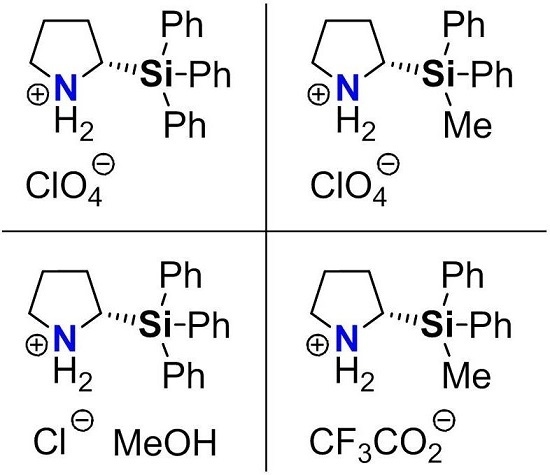
Molecular Structures of Enantiomerically-Pure (S)-2-(Triphenylsilyl)- and (S)-2-(Methyldiphenylsilyl)pyrrolidinium Salts
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Details
3.1. Synthetic Methods
3.2. X-ray Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bauer, J.O.; Stiller, J.; Marqués-López, E.; Strohfeldt, K.; Christmann, M.; Strohmann, C. Silyl-modified analogues of 2-tritylpyrrolidine: Synthesis and applications in asymmetric organocatalysis. Chem. Eur. J. 2010, 16, 12553–12558. [Google Scholar] [CrossRef] [PubMed]
- Husmann, R.; Jörres, M.; Raabe, G.; Bolm, C. Silylated pyrrolidines as catalysts for asymmetric Michael additions of aldehydes to nitroolefins. Chem. Eur. J. 2010, 16, 12549–12552. [Google Scholar] [CrossRef] [PubMed]
- Jentzsch, K.I.; Min, T.; Etcheson, J.I.; Fettinger, J.C.; Franz, A.K. Silyl fluoride electrophiles for the enantioselective synthesis of silylated pyrrolidine catalysts. J. Org. Chem. 2011, 76, 7065–7075. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Fettinger, J.C.; Franz, A.K. Enantiocontrol with a hydrogen-bond directing pyrrolidinylsilanol catalyst. ACS Catal. 2012, 2, 1661–1666. [Google Scholar] [CrossRef]
- Kerrick, S.T.; Beak, P. Asymmetric deprotonations: Enantioselective syntheses of 2-substituted (tert-butoxycarbonyl)pyrrolidines. J. Am. Chem. Soc. 1991, 113, 9708–9710. [Google Scholar] [CrossRef]
- Strohfeldt, K.; Seibel, T.; Wich, P.; Strohmann, C. Synthesis and reactivity of an enantiomerically pure N-methyl-2-silyl-substituted pyrrolidine. In Organosilicon Chemistry VI: From Molecules to Materials; Auner, N., Weis, J., Eds.; Wiley-VCH: Weinheim, Germany, 2005; Volume 1, pp. 488–494. [Google Scholar]
- Kano, T.; Mii, H.; Maruoka, K. Direct asymmetric benzoyloxylation of aldehydes catalyzed by 2-tritylpyrrolidine. J. Am. Chem. Soc. 2009, 131, 3450–3451. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, H.; An, F.; Mayer, P.; Ofial, A.R.; Lakhdar, S.; Mayr, H. Structures and reactivities of 2-trityl- and 2-(triphenylsilyl)pyrrolidine-derived enamines: Evidence for negative hyperconjugation with the trityl group. J. Am. Chem. Soc. 2014, 136, 14263–14269. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.O.; Strohmann, C. tert-Butoxytriphenylsilane. Acta Crystallogr. 2010, E66, o461–o462. [Google Scholar] [CrossRef] [PubMed]
- Brendler, E.; Heine, T.; Seichter, W.; Wagler, J.; Witter, R. 29Si NMR shielding tensors in triphenylsilanes—29Si solid state NMR experiments and DFT-IGLO calculations. Z. Anorg. Allg. Chem. 2012, 638, 935–944. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97, a Program for the Solution of Crystal Structures; Universität Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, a Program for Crystal Structure Refinement; Universität Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Flack, H.D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690. [Google Scholar] [CrossRef] [PubMed]





| Compound | (S)-1·HClO4 | (S)-1·HCl·MeOH | (S)-2·HClO4 | (S)-2·TFA |
|---|---|---|---|---|
| Empirical formula | C22H24ClNO4Si | C23H28ClNOSi | C17H22ClNO4Si | C19H22F3NO2Si |
| Formula weight [g·mol−1] | 429.96 | 398.00 | 367.90 | 381.47 |
| Crystal system | Monoclinic | Orthorhombic | Monoclinic | Orthorhombic |
| Space group | P21 | P21 21 21 | P21 | P21 21 21 |
| a [Å] | 8.1535(2) | 7.2981(3) | 9.6006(6) | 9.5760(14) |
| b [Å] | 7.8737(2) | 11.7818(5) | 14.2054(8) | 9.7100(17) |
| c [Å] | 16.9410(4) | 25.1513(11) | 13.3890(8) | 41.176(4) |
| β [°] | 100.780(2) | 90 | 91.537(6) | 90 |
| Volume [Å3] | 1068.39(5) | 2162.63(16) | 1825.34(19) | 3828.7(9) |
| Z | 2 | 4 | 4 | 8 |
| Density (calculated) ρ [g·cm−3] | 1.337 | 1.222 | 1.339 | 1.324 |
| Absorption coefficient μ [mm−1] | 0.263 | 0.245 | 0.295 | 0.163 |
| F(000) | 452 | 848 | 776 | 1600 |
| Crystal size [mm3] | 0.20 × 0.20 × 0.10 | 0.40 × 0.30 × 0.20 | 0.20 × 0.20 × 0.10 | 0.40 × 0.20 × 0.10 |
| Theta range for data collection θ [°] | 2.45–25.99 | 2.37–26.00 | 2.56–26.00 | 2.18–25.00 |
| Index ranges | –10 ≤ h ≤ 10 | –9 ≤ h ≤ 9 | –11 ≤ h ≤ 11 | –11 ≤ h ≤ 11 |
| −9 ≤ k ≤ 9 | −14 ≤ k ≤ 14 | −17 ≤ k ≤ 17 | −11 ≤ k ≤ 10 | |
| −20 ≤ l ≤ 20 | −31 ≤ l ≤ 30 | −16 ≤ l ≤ 16 | −47 ≤ l ≤ 48 | |
| Reflections collected | 46,918 | 16,132 | 28,993 | 41,441 |
| Independent reflections | 4181 (Rint = 0.0369) | 4244 (Rint = 0.0388) | 7177 (Rint = 0.0540) | 6661 (Rint = 0.0425) |
| Completeness to θ | 100.0% (θ = 25.99°) | 99.9% (θ = 26.00°) | 99.9% (θ = 26.00°) | 99.3% (θ = 25.00°) |
| Max. and min. transmission | 0.9742 and 0.9493 | 0.9527 and 0.9085 | 0.9711 and 0.9433 | 0.9681 and 0.9376 |
| Data/restraints/parameters | 4181/1/270 | 4244/0/254 | 7177/1/435 | 6661/0/566 |
| Goodness-of-fit on F2 | 1.000 | 1.000 | 1.000 | 1.000 |
| Final R indices [I > 2σ(I)] | R1 = 0.0237, wR2 = 0.0620 | R1 = 0.0337, wR2 = 0.0682 | R1 = 0.0596, wR2 = 0.1683 | R1 = 0.0340, wR2 = 0.0572 |
| R indices (all data) | R1 = 0.0257, wR2 = 0.0624 | R1 = 0.0459, wR2 = 0.0700 | R1 = 0.0809, wR2 = 0.1745 | R1 = 0.0539, wR2 = 0.0592 |
| Absolute structure parameter (Flack parameter) | 0.01(4) | 0.01(6) | 0.08(9) | 0.02(8) |
| Largest diff. peak and hole [e·Å−3] | 0.207 and −0.228 | 0.424 and −0.252 | 0.447 and −0.309 | 0.260 and −0.197 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, J.O.; Strohmann, C. Molecular Structures of Enantiomerically-Pure (S)-2-(Triphenylsilyl)- and (S)-2-(Methyldiphenylsilyl)pyrrolidinium Salts. Inorganics 2017, 5, 88. https://doi.org/10.3390/inorganics5040088
Bauer JO, Strohmann C. Molecular Structures of Enantiomerically-Pure (S)-2-(Triphenylsilyl)- and (S)-2-(Methyldiphenylsilyl)pyrrolidinium Salts. Inorganics. 2017; 5(4):88. https://doi.org/10.3390/inorganics5040088
Chicago/Turabian StyleBauer, Jonathan O., and Carsten Strohmann. 2017. "Molecular Structures of Enantiomerically-Pure (S)-2-(Triphenylsilyl)- and (S)-2-(Methyldiphenylsilyl)pyrrolidinium Salts" Inorganics 5, no. 4: 88. https://doi.org/10.3390/inorganics5040088
APA StyleBauer, J. O., & Strohmann, C. (2017). Molecular Structures of Enantiomerically-Pure (S)-2-(Triphenylsilyl)- and (S)-2-(Methyldiphenylsilyl)pyrrolidinium Salts. Inorganics, 5(4), 88. https://doi.org/10.3390/inorganics5040088