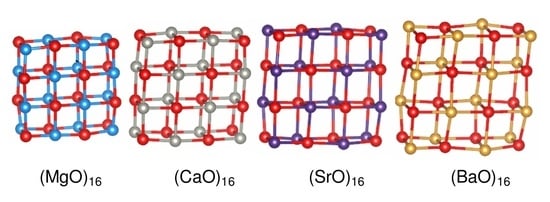
3.1. Structures of (MO) Clusters ( and 24)
Table 1 shows structures and energies for PBEsol LM, which are found to be ranked within the top 3 lowest energy LM for at least one of the clusters (alkaline earth oxide, M = Mg, Ca, Sr and Ba and sizes
to 12); the energies (per MO unit) are relative to the corresponding GM. These structures are labelled with letters to indicate their energy ranking for (MgO)
, (CaO)
, (SrO)
and (BaO)
(in that order), A being the GM, B the second-lowest, etc.; for example, a structure which is the GM for all four compounds will be labelled AAAA and one which is the GM for (CaO)
and third-lowest energy for the other three compounds is labeled CACC. If a structure is not among the three most stable ones for a given oxide, the designated letter will be X.
For sizes , it is possible to construct cuboid clusters composed of face sharing cuboid units and hexagonal barrel shaped clusters composed of k parallel hexagonal rings. Comparing the configurations found for GM, the most striking observation is that calcium oxide, strontium oxide and barium oxide prefer cuboid cuts from the NaCl-rocksalt structure for sizes , while the barrel is the predicted GM configuration for magnesium oxide.
Considering the top three structures for each size, both the rocksalt cuts (cuboid and non-cuboid clusters composed of face-sharing cuboid building blocks ) and barrel motifs appear for all four oxides. One structural motif that is seen more frequently for compounds like zinc oxide is the so called bubble; atoms are all three coordinated and, typically, there are six tetragonal and hexagonal faces. Although the GM for sizes 4 to 7 (the cube-, drum- and clam-like configurations, respectively) can be classified as bubbles, the number of bubble configurations that are ranked within the top three typically decreases with increasing cation size in the alkali earth oxides as these become less stable with respect to the GM with increasing cluster size. We identify configurations 4-AAAA, 5-CBAC, 7-AAAB, 8-AXXC, 9-BCXX, 10-CXXX, 11-CXXX and 12-CXXX (see also 16-CXXX) to be bubble LM for (MgO). We can already identify from the labels that far less bubble LM are in the three lowest energy configurations for (CaO) (4 total), (SrO) (only 3) and (BaO) (4 total); all these configurations will be very small clusters where there are smaller numbers of total available configurations.
Comparing the nanoclusters of the four alkaline earth oxides, magnesium oxide is more likely to adopt the more open structures composed of the lower three coordinated sites than the six-coordinated sites found within the bulk phase. The three lowest energy configurations for (BaO), on the other hand, nicely demonstrate this typical trend: the order of stability increases with an increase in the total number of bonds (or average coordination number) and, simultaneously, the order of stability increases with an increase in density (decrease in openness, or number of hexagonal faces that become an edge-sharing pair of tetragonal faces). Configuration 8-BAAA is a (counting atoms) cuboid bulk cut and can be considered as two parallel atomic layers, just one bond length apart, of three tetragonal rings. Configuration 8-XBBB has one less bond in each of the two layers, and represents structures composed of two parallel atomic layers, still only one bond length apart, that do not only contain tetragons (in this example, each of the two sequences of three tetragonal faces become one tetragonal and one hexagonal face). Configuration 8-AXXC has a further bond reduction between parallel layers; the original 8-BAAA configuration has four sides of three tetragonal faces, each becoming one tetragonal and one hexagonal face, and in doing so creating a larger void in the centre of the cluster (than the combined volume within the (BaO) cuboid and (BaO) drum, as in configuration 8-XBBB). Only for (MgO), the compound with the smallest cation, is the bubble structure the GM and the intermediate 8-XBBB configuration found for (BaO) is unstable for MgO, indicating that perhaps there are more stable LM overall for larger-cation clusters.
For
, all oxides’ GMs except barium oxide’s adopt a bulk cut structure—more specifically, all adopt a
bulk cut with one corner atom removed but (MgO)
is different, in that it adopts the one with M in the centre, while (CaO)
and (SrO)
show a preference for O in the centre. In the case of (BaO)
, if the initial starting DFT geometry resembled the rocksalt cut similar to that shown for (CaO)
, then the resulting relaxed configuration would be the GM amorphous structure shown in
Table 2 for (BaO)
(13-XXXA). For both larger and smaller BaO clusters, however, bulk cuts are found (for
, the bulk cut cluster is ranked third). For
, all GM are bulk cuts apart from magnesium oxide where, given the
cuboid fragment is now less stable than the
drum, the GM is composed of two parallel atomic layers, one bond length apart, each containing one hexagonal ring (see discussion above about (BaO)
LM). In the case of
, again all except magnesium oxide adopt the rocksalt cut structure, with (MgO)
forming a long hexagonal barrel. In
clusters, a structure that is partially composed of a 8-membered ring barrel (17-ACXX) is found to be the GM for (MgO)
and also the third-lowest energy structure of the calcium oxide cluster.
Cuboid clusters are only a subset of LM configurations that resemble cuts from the rocksalt structure. Some insight can be gained by comparing the additional exposed surfaces of our non-cuboid bulk-cut clusters with those of the perfect cuboid cuts. In the latter, we only have six surfaces that are effectively finite (001) bulk-terminated surfaces. In the case of 11-XAAC and 15-XBBX, the additional face is equivalent to the (011) bulk-terminated surface while for 13-AVXX and 13-BAAX, the equivalent surfaces are (111). This leads to 3-coordinated non-corner atoms, which appear to be favoured less when larger cations are present. For , the difference between the GM configuration for (MgO) and the other three oxides is more noticeable as the (MgO) GM is the hexagonal barrel composed of five stacked hexagonal rings, rather than a bulk cut from the rocksalt phase. The cuboid for , composed of atoms, is the GM for (CaO), (SrO) and (BaO) and second-lowest for (MgO); the (011) terminated bulk cut 15-XBBX is only a top 3 structure for (CaO) and (SrO). For and, most importantly and , as at these sizes it is again possible to form a barrel motif, magnesium oxide clusters also preferentially adopt a cuboid, rocksalt cut structure. The barrel is the second-lowest energy structure for (MgO) and while it is not ranked in the top 10 structures for (MgO), many of the lowest-energy structures are partially barrel-like. The case of is also noteworthy as there are two possible cuboid configurations composed of either or atoms. This will be discussed in more detail below.
The stability of a configuration will increase with increasing average coordination but decrease with any resulting increase in strain. A resulting dipole across the cluster can also affect the stability of that configuration, and as we will see, the degree to which each cation can polarize to screen, or counter this, will likewise influence the ranking of configurations. Of these factors, coordination number and the calculation of dipoles are straightforward to compute, so this data, along with the second-order energy differences, are now reported.
3.2. Second-Order Energies and Structural Motifs
The second-order energy differences between the GM clusters for
to 17 are plotted below. The second-order energy difference of a cluster size
n is defined as
where
is the energy of the lowest-energy structure of size
n for a given compound.
provides a measure of relative stability of cluster sizes. As
is the GM of a particular size
n, the minima of
represent local size stabilities.
These second-order energy differences are presented here as two figures,
Figure 1 for magnesium oxide clusters and
Figure 2 for clusters of the other three compounds, as their second-order energies were very similar. All second-order energy differences are also tabulated in
Table 3.
The trend discussed in
Section 3.1 for magnesium oxide clusters is echoed in
Figure 1: the structural motif adopted by most the structures with negative second-order energies is that of the barrel (
and 15). The
cuboid rocksalt cut GM configuration of (MgO)
, 16-AAAA, is relatively more stable than its neighbours.
Figure 2 shows the second-order energy differences for GM nanoclusters with M = Ca, Sr, Ba. The structures for
n with negative second-order energies are typically the same for all three, so only ball and stick models of the SrO configurations are shown. There are examples where the GM configuration is different for the compound with the largest cation (
and 18) and where all three oxides differ (
and 17). All GM configurations, however, with an even value of
n or with a value of
have a negative or close to zero second-order energy for all three compounds, which corresponds to sizes where the construction of a cuboid bulk cut is feasible. The only non-cuboid structure (intermediate between the
(
) and
(
) cuboids) appearing here is the
bulk cut, with negative second-order energies for (CaO)
and (SrO)
only. Typically, the magnitude of the second-order energies is smallest for GM clusters of (BaO)
and largest for (CaO)
.
For sizes to 10, the GM structures are cuboids with increasing surface area ( tetragons) or decreasing surface area per atom (, , ), but with the middle size cuboid maximising its volume per atom (, and cuboids, respectively). However, from R analysis of surface area vs. energy of structures, we found little to no correlation. Sizes to 16 are another three bulk cut structures (although in the case of not cuboid), which all have negative second-order energies for some of the metal oxides, but not all.
3.3. Coordination Numbers and Deformation
The average coordination number in GM clusters against
n is plotted in
Figure 3. The average coordination number is typically lowest (where the same structural motif is not adopted for the GM configurations across all compounds) in magnesium oxide GM nanoclusters. This is unsurprising given that the coordination number will inherently be lower for barrel shapes than in rocksalt cuts as the maximum coordination number in a barrel is 4, compared to 6 in the case of an atom in the centre of a cuboid rocksalt cut. As discussed above, some higher energy LM structures (the second- and third-lowest energy LM) of (MgO)
clusters tend to have an even lower average coordination number; the perfect bubble structures (see, for example, structures 11-CXXX and 12-CXXX) have atoms that are all three-coordinated. Configurations can also be constructed from tetragonal face sharing
drums and
n = 9, 12, 15... barrels, which would increase the average coordination. The smallest of these (
composed of two drums with four four-coordinated atoms (10-XXXC);
n = 13 composed of three drums with eight four-coordinated atoms;
n = 15 composed of two
barrels with two five-coordinated atoms) are either less stable or actually unstable. For example, the
n =10 cluster can either open out to form a bubble or collapse to form the
cuboid (10-AAAA). However, when combined with face sharing
n = 4 cuboid clusters, a number of the LM shown in
Table 1 and
Table 2 can be constructed. When the barium oxide clusters’ GM is different to that of (CaO)
and (SrO)
, its average coordination number is also typically smaller; here, this occurs when
n is odd, i.e., sizes 11, 13, and 17, the latter two being armorphous configurations.
When looking at sizes to 10 for clusters containing one of the three largest cations, the second-order energy makes an interesting case to examine more closely. The compound with the smallest cation, Ca, favours relatively strongly, and has a positive second-order energy for . In stark contrast, the compound with the largest cation, Ba, has a positive value for and favours and 10. Completing this trend, although closer to the value for calcium oxide, for strontium oxide lies in between those calculated for the oxides of calcium and barium. The competing factors in these cuboids are the average number of dangling bonds and therefore to the average coordination number, which also has a minimum for , and the dipole moment, which is effectively zero for and 10 but non-zero for , as this has planes that contain an odd number of atoms. Hence, it appears that it is more favourable for Ca atoms to maximise their average coordination number, whilst the creation of a dipole appears most unfavourable for barium oxide. A similar trend becomes apparent with to 16; the polar cluster is most stable for calcium oxide clusters and least stable for barium oxide ones.
For clusters containing the three larger alkaline cations, it is also apparent upon comparing
Figure 2 and
Figure 3 that the local minima in the average coordination numbers (
) match the local maxima in the second-order energies. Alternatively, the GM structures that are energetically more stable than their neighbouring sizes tend to have a higher average coordination number. In the case of (MgO)
, a lower average coordination number is found for the barrel-shaped GM clusters with a size that is more stable than the average of its nearest neighbours (i.e., sizes
and 15). This trend ends once the size of the cluster passes the transition point for bulk-cut GM.
Examining why and how exactly magnesium oxide is different in its preference for barrel shapes in smaller clusters, it is found that the cuboid GM configuration of (MgO) is relatively more stable than its neighbours, which includes the (MgO) barrel. This is indicative that the bulk transition, where magnesium oxide clusters start adopting bulk-like structures as opposed to barrels, occurs around this size. However, we note that, in the case of , the cuboid configuration, although ranked second, is still eV higher in energy than the barrel. The local minimum of at rather than 15 is perhaps more to do with the relatively low stability of , a size where no perfect barrel nor cuboid cut is possible, and the perfect bubbles of this size have low symmetry. Note that the GM for (MgO) is a defective (along one edge) bulk cut, whereas the GM for (MgO) is a defective (along one of the shortest edges) bulk cut.
It has been previously reported [
24] that the dipole moment for the
(MgO)
cuboid configuration is expected to be rather high since, as discussed above, it contains planes that have an odd number of atoms, so we looked at the dipoles reported in our DFT outputs. Note that there is no significant dipole moment for the perfect
cuboid configurations, and that the
configurations were much higher in energy for all four oxides and should also not have a significant dipole moment. The dipole moments for all four
clusters with the
cuboid configuration are reported in
Table 4; the magnesium oxide, calcium oxide and strontium oxide structures are the GM but barium oxide preferred the non-polar structure. The dipole moment decreases with an increase in cation size, counter to naïve arguments based on interatomic distances.
The degree of polarisation has previously been related to the acuteness of the cation–anion–cation bond angles in nanoclusters [
23]—trends in both this and coordination numbers map quite well onto these angles for small clusters. We compared the angles between adjacent short contacts we found for
to four GM structures to those found the corresponding ideal shapes (90 degrees for the
square, 120 for the
hexagon and 90 again for the
cube). The absolute deviations of the angles are shown in
Figure 4. For reference as to how this looks in specific clusters, see the
structures in
Table 1, where the (MgO)
and (BaO)
structures are deformed in opposite ways.
In the case of magnesium oxide, the Mg–O–Mg angles were more acute than the expected angle (and correspondingly O–Mg–O angles were wider). For all other cases, M–O–M angles were wider than expected, with a clear overall trend of this angle opening up and the O–M–O angle closing with an increase in cation size. The closest to ideal angles is therefore calcium oxide.
3.4. Dipole Moments
Although for the same polar structure and assuming formal point charges you would get a greater dipole moment when the spacing between charged layers is increased, it is evident from comparing the data in
Table 4 that the dipole moment decreases with cation size and actually drops by more than a factor of 2 between strontium oxide and barium oxide. Why, then, is the polar
cuboid cluster the GM for three of the alkaline oxides and not for barium oxide? Assuming formal point charges of
is clearly inaccurate particularly as the cations have very different polarizabilities: Ba
ions are much more polarizable than the other metal ions [
30] (actually the polarizability of Ba
is about twice that of Sr
). Due to the higher polarizability, the orbitals can typically deform more easily and more mixing occurs. Through this, the dipole across the cluster can be more readily reduced for barium oxide clusters than for the other alkaline oxide polar cuboid clusters. However, one must not forget the influence of other factors; for example, how strong the penalty for low-coordinated atoms is. This penalty is presumably stronger in BaO given the larger cation can potentially accommodate a higher coordination and, therefore, acts to reverse what is found given the average coordination of the GM for (BaO)
is lower than that of the GM predicted for the other three. Comparing the atom sites in the two GM
bulk cuts, although they have the same number of corner sites, the non-polar cut that is adopted by (BaO)
has four more edge sites (and fewer face and bulk sites). Thus, the average atom in the non-polar cut has more space for electronic polarisation and this effect outweighs the combination of other factors to result in the non-polar cut to be the GM for (BaO)
(but clearly not dominant enough for the
cuboid bulk cut to be ranked first).
Connecting the polarisation data with acuteness of M–O–M angles, the trends in both dipole moment and coordination numbers are reflected here as they were for lanthanum fluoride clusters [
23]. Additionally, the acuteness of M–O–M angles may also shed light on the preference for different bulk cuts for
(see
Table 2). It also appears that calcium oxide clusters might be expected to also adopt structures that are least distorted from ideal rocksalt cuts, in line with its favourable energetics even for non-cuboid cuts. This result is in line with expectations based on polarizabilities. In the case of magnesium oxide, the anion is much more polarizable than the cation, and therefore more likely to “move out”. As the cation size is increased, the cation polarizability is also increased, and therefore the larger cations are more likely to pucker out. This does suggest that these two effects balance out most effectively for calcium oxide. Earlier the second-order energies for
and
were shown to increase down the group (CaO→SrO→BaO), suggesting that the polar structure is relatively more stable if the cation is less polarizable and the bonds are shorter. The degree of freedom, or space, for electronic polarisation provides a more satisfactory explanation as to why the
cuboid bulk cut is predicted to be less stable than the average of the neighbouring sized non-polar
and
cuboid bulk cuts if the cations are barium, whereas the reverse is predicted for either calcium or strontium. Moreover, the same argument can be used to explain relative change in the rank of the polar
cuboid, 15-BAAA, bulk cut; its second-order energy is not lower than that of the
non-polar bulk cut for any oxide, and the
cuboid cut structure is relatively most stable with calcium as the cation and least stable with barium. However, comparing to the
non-cuboid cut reveals that its polar nature is penalised most strongly for BaO, while it is preferred the most over the 14-XAAA structure by CaO.
3.7. Comparison with Previous Results
One experiment that our results can be compared against is mass spectrometry, but this needs to be considered with great care since experiments will typically involve charged clusters, whereas we have only examined neutral clusters. Moreover, mass spectrometry only gives insight into likely cluster sizes and sub-unit sizes, rather than the structures of the clusters. However, it can nevertheless aid in the interpretation and validation of computational results. Mass spectrometric studies on the alkaline earth oxides indicate especially high stability of the
magnesium oxide fragments [
7], but
(MgO)
peaks have also been detected alongside the
ones [
8] (albeit weaker). For calcium oxide,
fragments seemed to appear more frequently [
9], indicating that four atom building blocks are perhaps favoured. This correlates strongly with the structures, which had low second-order energies: in the case of CaO, they were all
bulk cuts, while, for (MgO)
, the
fragments correspond to bulk cuts (they can be interpreted as square building units) and the
fragments are from clusters formed of parallel hexagonal rings, which form barrel-like configurations.
The trends for magnesium oxide clusters (as well as the general region where the bulk transition occurs) are also generally in line with previous computational results [
10,
11,
12,
13,
14,
15,
16,
17], which typically report clusters of selected sizes or sizes up to
. A recent study on (CaO)
clusters [
19] data-mined from (MgO)
geometries also found that cuboid structures were energetically more favourable for calcium oxide clusters than for magnesium oxide clusters. Although their study also included constructed cuboid cuts, barrels and multiple barrels, they do not report all non-cuboid bulk cuts, including our GM found for
(11-XAAC) and 14 (14-XAAA) as well as two LM, 11-XCCX and 13-ABXX, reported here in
Table 1 and
Table 2. In their original magnesium oxide study [
18], which used a tree growth-hybrid genetic algorithm [
31], they reported the same GM structures that were found here for all sizes except for
. Given we have employed a different density functional, it is not surprising that there is some discrepancy. Our PBEsol-GM configuration, 11-AXXX, is reported as their second lowest B3LYP-LM (147 meV) higher than their B3LYP-GM), whereas their GM configuration (cf. 11-XXXB) is 7.9 meV/MgO higher in energy, as measured using PBEsol, than our GM. Other differences are also found in the low-lying LM; for example, for size
, we report three rather than two non-planar candidate structures. Particular configurations may not be reported in one of the studies because of a large change in their rank upon the change in the choice of the functional (in an extreme case, the LM may become unstable) or because the configurations were genuinely missed by one of the studies (which is more likely to occur for large
n). In Ref. [
19], a number of different energy functions, including CCSD(T), were compared by calculating the energy differences between the smallest B3LYP GM configuration and their larger sized B3LYP tentative GM. The main premise in this comparison is that the CCSD(T) level of theory provides the best available reference to date, which crucially depends on inclusion of diffuse and trained polarisation functions in the basis sets for an accurate description of the polarisation effects. These features are important in dealing with clusters of ionic systems that expose under-coordinated anionic atoms. The DFT methods in fact converge much better with the basis set and the numerical basis functions with accurate behaviour in the orbital tails and near cusp regions (employed here) are practically at the basis set limit. To aid future comparisons between studies, we have uploaded our configurations into the HIVE online database [
3]. Importantly for our predictions as to which configurations are more likely to be isolated in experiments, we have focused on finding GM that are much lower in energy than LM of similar size compared to the energy change that is likely to occur when changing the energy functional.
One of the studies on (MgO)
mentioned earlier, which also includes comparison with IR spectra [
21], goes up to
; they suggest that the experimentally found structure, which is also the most stable structure thermodynamically for
, is not the bulk cut, but rather the structure which for us is second-lowest in energy (16-BCBC). However, they go on to suggest that both structures are likely to form in experiments and that their data is in line with this. Our data supports this also. When two structures are very close in energy, inaccuracies in choice of functional and basis set are likely to change their ranking, but both structures will still be likely to form in experiment.
Comparison with other ionic compounds with the same stoichiometry can give additional insight. For example, in previous computational studies of alkali halides, one paper presented DFT results for barrel and rocksalt cut structures for small (MX)
clusters of alkali halides lithium fluoride, sodium chloride and potassium bromide [
32]; these calculations suggested that for all three the lower energy structure of the two was the barrel one with
. The authors described elsewhere a corresponding result for (LiF)
[
33]. In all of these compounds, the anion’s ionic radius is much larger than the cation’s [
34], making the most similar to those of magnesium oxide in terms of this ratio as well as structure.
An alkali halide that is perhaps of interest in comparison to barium oxide in particular is potassium fluoride: anions are ordinarily much larger in radius than cations due to the outermost electrons being less tightly bound (as their nuclear charge is relatively smaller and more shielded), but in both barium oxide and potassium fluoride, the cations are large and the anions small, leading to a cation:anion radius ratio of ∼1 [
34]. Previous research has shown that in KF there is also a preference for the bulk cut structure [
12]. Combining this with our results for the alkaline earth oxides, it appears that, overall, the relative stability of rocksalt cuts (compared to barrels) is a higher structure when the cation size is increased and/or anion size is decreased.
As reported here, the dipole moment of equivalent clusters decreases with an increase in cation size, and the similarity in structures for similar anion:cation ratios in alkali halides points toward trends in various properties being perhaps more universal.







 Mg,
Mg,  Ca,
Ca,  Sr,
Sr,  Ba and
Ba and  . A stick is shown if an oxygen anion is within the first coordination shell of a cation (typically less than 2.5 Å for Mg and 2.85 Å for Ba).
. A stick is shown if an oxygen anion is within the first coordination shell of a cation (typically less than 2.5 Å for Mg and 2.85 Å for Ba).
 Mg,
Mg,  Ca,
Ca,  Sr,
Sr,  Ba and
Ba and  . A stick is shown if an oxygen anion is within the first coordination shell of a cation (typically less than 2.5 Å for Mg and 2.85 Å for Ba).
. A stick is shown if an oxygen anion is within the first coordination shell of a cation (typically less than 2.5 Å for Mg and 2.85 Å for Ba).












































 Mg,
Mg,  Ca,
Ca,  Sr,
Sr,  Ba and
Ba and  . A stick is shown if an oxygen anion is within the first coordination shell of a cation (typically less than 2.5 Å for Mg and 2.85 Å for Ba). In the case of , the letters in brackets denote PBESol0 results for (BaO).
. A stick is shown if an oxygen anion is within the first coordination shell of a cation (typically less than 2.5 Å for Mg and 2.85 Å for Ba). In the case of , the letters in brackets denote PBESol0 results for (BaO).
 Mg,
Mg,  Ca,
Ca,  Sr,
Sr,  Ba and
Ba and  . A stick is shown if an oxygen anion is within the first coordination shell of a cation (typically less than 2.5 Å for Mg and 2.85 Å for Ba). In the case of , the letters in brackets denote PBESol0 results for (BaO).
. A stick is shown if an oxygen anion is within the first coordination shell of a cation (typically less than 2.5 Å for Mg and 2.85 Å for Ba). In the case of , the letters in brackets denote PBESol0 results for (BaO).