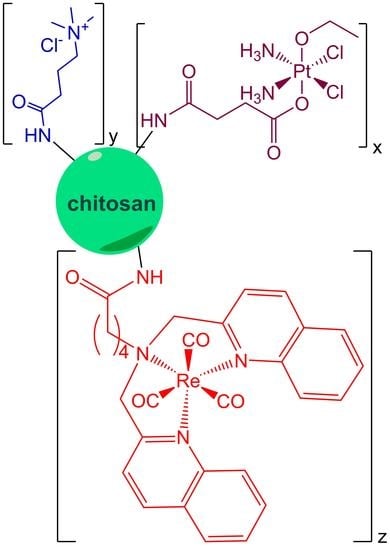
Pt(IV)/Re(I) Chitosan Conjugates as a Flexible Platform for the Transport of Therapeutic and/or Diagnostic Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the Conjugates
2.2. Antiproliferative Activity and Cellular
3. Materials and Methods
3.1. General Procedures
3.2. Titrations: Deacetylation Degree of Chitosan
3.3. Synthesis of the Chitosan Modified with (3-Carboxypropyl)trimethylammonium Chloride (C1)
3.4. Loading of the Pt(IV) Complexes on Chitosan (C12)
3.5. Coupling of the Modified Chitosan Conjugate C12 with the Ligand 5-[bis(Quinolin-2-ylmethyl)amino]pentanoic Acid, 3 (Conjugate C123)
3.6. Chelation Reaction of Conjugate C123 with the Re(I) Complex (C1234)
3.7. Solution Behaviour
3.8. Antiproliferative Activity
3.9. Cellular Uptake
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Conjugate | Reaction Time | Experimental % Pt Loading | µmol Pt g−1 Chitosan |
|---|---|---|---|
| C12a | 2 h | 0.78 ± 0.02 | 40.0 ± 0.9 |
| C12b | 4 h | 0.68 ± 0.12 | 35.2 ± 6.0 |
| C12c | 6 h | 0.86 ± 0.03 | 44.1 ± 1.5 |
| C12d | 8 h | 0.61 ± 0.08 | 31.4 ± 3.9 |
| C12e | 24 h | 0.99 ± 0.22 | 51.3 ± 11.2 |
| Conjugate | Theoretical Maximum % Pt Loading | Experimental % Pt Loading | µmol Pt g−1 Chitosan |
|---|---|---|---|
| C12f | 1 | 0.45 ± 0.03 | 23.2 ± 1.5 |
| C12b | 2 | 0.68 ± 0.12 | 35.2 ± 6.0 |
| C12g | 5 | 1.38 ± 0.12 | 71.0 ± 6.2 |
| C12h | 10 | 2.63 ± 0.05 | 135.0 ± 0.3 |
References
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef] [PubMed]
- Apps, M.G.; Choi, E.H.Y.; Wheate, N.J. The state-of-play and future of platinum drugs. Endocr. Relat Cancer 2015, 22, R219–R233. [Google Scholar] [PubMed]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Jain, R.K. Design considerations for nanotherapeutics in oncology. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.S.; Sadler, P.J. Targeted delivery of platinum-based anticancer complexes. Curr. Opin. Chem. Biol. 2013, 17, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.A.; Xiao, H.H.; Li, C.X.; Dai, Y.L.; Cheng, Z.Y.; Hou, Z.Y.; Lin, J. Inorganic nanocarriers for platinum drug delivery. Mater. Today 2015, 18, 554–564. [Google Scholar] [CrossRef]
- Maldonado, C.R.; Salassa, L.; Gomez-Blanco, N.; Mareque-Rivas, J.C. Nano-functionalization of metal complexes for molecular imaging and anticancer therapy. Coord. Chem. Rev. 2013, 257, 2668–2688. [Google Scholar] [CrossRef]
- Kim, J.; Pramanick, S.; Lee, D.; Park, H.; Kim, W.J. Polymeric biomaterials for the delivery of platinum-based anticancer drugs. Biomater. Sci. 2015, 3, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- Massaguer, A.; Gonzalez-Canto, A.; Escribano, E.; Barrabes, S.; Artigas, G.; Moreno, V.; Marchan, V. Integrin-targeted delivery into cancer cells of a Pt(IV) pro-drug through conjugation to RGD-containing peptides. Dalton Trans. 2015, 44, 202–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, X.; Guo, Z. Functionalization of platinum complexes for biomedical applications. Acc. Chem. Res. 2015, 48, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.A.; Prashar, S.; Shreaz, S.; Gomez-Ruiz, S. Nanostructured materials functionalized with metal complexes: In search of alternatives for administering anticancer metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- Gabano, E.; Ravera, M.; Colangelo, D.; Osella, D. Bioinorganic chemistry: The study of the fate of platinum-based antitumour drugs. Cur. Chem. Biol. 2007, 1, 278–289. [Google Scholar]
- Gabano, E.; Ravera, M.; Osella, D. The drug targeting and delivery approach applied to Pt-antitumour complexes: A coordination point of view. Cur. Med. Chem. 2009, 16, 4544–4580. [Google Scholar] [CrossRef]
- Hall, M.D.; Mellor, H.R.; Callaghan, R.; Hambley, T.W. Basis for design and development of Platinum(IV) anticancer complexes. J. Med. Chem. 2007, 50, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Graf, N.; Lippard, S.J. Redox activation of metal-based prodrugs as a strategy for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Wexselblatt, E.; Gibson, D. What do we know about the reduction of Pt(IV) pro-drugs? J. Inorg. Biochem. 2012, 117, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Gianferrara, T.; Spagnul, C.; Alberto, R.; Gasser, G.; Ferrari, S.; Pierroz, V.; Bergamo, A.; Alessio, E. Towards matched pairs of Porphyrin-ReI/99mTcI conjugates that combine photodynamic activity with fluorescence and radio imaging. ChemMedChem 2014, 9, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Quental, l.; Raposinho, P.; Mendes, F.; Santos, I.; Navarro-Ranninger, C.; Alvarez-Valdes, A.; Huang, H.; Chao, H.; Rubbiani, R.; Gasser, G.; et al. Combining imaging and anticancer properties with new heterobimetallic Pt(II)/M(I) (M = Re, 99mTc) complexes. Dalton Trans. 2017, 46, 14523–14536. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.; Esteves, T.; Gano, L.; Raposinho, P.D.; Paulo, A.; Santos, I. Synthesis, characterization and biological evaluation of tricarbonyl M(I) (M = Re, Tc-99m) complexes functionalized with melanin-binding pharmacophores. New J. Chem. 2010, 34, 2564–2578. [Google Scholar] [CrossRef]
- Can, D.; Spingler, B.; Schmutz, P.; Mendes, F.; Raposinho, P.; Fernandes, C.; Carta, F.; Innocenti, A.; Santos, I.; Supuran, C.T.; et al. (Cp-R)M(CO)3 (M = Re or 99mTc) arylsulfonamide, arylsulfamide, and arylsulfamate conjugates for selective targeting of human carbonic anhydrase IX. Angew. Chem. Int. Ed. 2012, 51, 3354–3357. [Google Scholar] [CrossRef] [PubMed]
- Leonidova, A.; Gasser, G. Underestimated potential of organometallic rhenium complexes as anticancer agents. ACS Chem. Biol. 2014, 9, 2180–2193. [Google Scholar] [CrossRef] [PubMed]
- Leonidova, A.; Pierroz, V.; Rubbiani, R.; Heier, J.; Ferrari, S.; Gasser, G. Towards cancer cell-specific phototoxic organometallic rhenium(I) complexes. Dalton Trans. 2014, 43, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Kitanovic, I.; Can, S.Z.; Alborzinia, H.; Kitanovic, A.; Pierroz, V.; Leonidova, A.; Pinto, A.; Spingler, B.; Ferrari, S.; Molteni, R.; et al. A deadly organometallic luminescent probe: Anticancer activity of a ReI bisquinoline complex. Chem. Eur. J. 2014, 20, 2496–2507. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Rubbiani, R.; Gasser, G.; Spingler, B. Visible-light-induced annihilation of tumor cells with platinum-porphyrin conjugates. Angew. Chem. Int. Ed. 2014, 53, 6938–6941. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan amphiphilic derivatives. Chemistry and applications. Cur. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Philippova, O.E.; Korchagina, E.V. Chitosan and its hydrophobic derivatives: Preparation and aggregation in dilute aqueous solutions. Polym. Sci. Ser. A 2012, 54, 552–572. [Google Scholar] [CrossRef]
- Riva, R.; Ragelle, H.; des Rieux, A.; Duhem, N.; Jérôme, C.; Préat, V. Chitosan and chitosan derivatives in drug delivery and tissue engineering. In Chitosan for Biomaterials II; Jayakumar, R., Prabaharan, M., Muzzarelli, R.A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 244, pp. 19–44. [Google Scholar]
- De Alvarenga, E.S.; de Oliveira, C.P.; Bellato, C.R. An approach to understanding the deacetylation degree of chitosan. Carbohydr. Polym. 2010, 80, 1155–1160. [Google Scholar] [CrossRef]
- Casettari, L.; Vllasaliu, D.; Castagnino, E.; Stolnik, S.; Howdle, S.; Illum, L. PEGylated chitosan derivatives: Synthesis, characterizations and pharmaceutical applications. Prog. Polym. Sci. 2012, 37, 659–685. [Google Scholar] [CrossRef]
- Sieval, A.B.; Thanou, M.; Kotze, A.F.; Verhoef, J.E.; Brussee, J.; Junginger, H.E. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr. Polym. 1998, 36, 157–165. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Sabaa, M.W.; El-Ghandour, A.H.; Abdel-Aziz, M.M.; Abdel-Gawad, O.F. Quaternized N-substituted carboxymethyl chitosan derivatives as antimicrobial agents. Int. J. Biol. Macromol. 2013, 60, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Domard, A.; Rinaudo, M.; Terrassin, C. New method for the quaternization of chitosan. Int. J. Biol. Macromol. 1986, 8, 105–107. [Google Scholar] [CrossRef]
- Ravera, M.; Gabano, E.; Zanellato, I.; Perin, E.; Arrais, A.; Osella, D. Functionalized nonporous silica nanoparticles as carriers for Pt(IV) anticancer prodrugs. Dalton Trans. 2016, 45, 17233–17240. [Google Scholar] [CrossRef] [PubMed]
- Ravera, M.; Perin, E.; Gabano, E.; Zanellato, I.; Panzarasa, G.; Sparnacci, K.; Laus, M.; Osella, D. Functional fluorescent nonporous silica nanoparticles as carriers for Pt(IV) anticancer prodrugs. J. Inorg. Biochem. 2015, 151, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Ravera, M.; Gabano, E.; Tinello, S.; Zanellato, I.; Osella, D. May glutamine addiction drive the delivery of antitumor cisplatin-based Pt(IV) prodrugs? J. Inorg. Biochem. 2017, 167, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ravera, M.; Gabano, E.; Zanellato, I.; Fregonese, F.; Pelosi, G.; Platts, J.A.; Osella, D. Antiproliferative activity of a series of cisplatin-based Pt(IV)-acetylamido/carboxylato prodrugs. Dalton Trans. 2016, 45, 5300–5309. [Google Scholar] [CrossRef] [PubMed]
- Alberto, R.; N’Dongo, H.P.; Clericuzio, M.; Bonetti, S.; Gabano, E.; Cassino, C.; Ravera, M.; Osella, D. Functionalized thymidine derivatives as carriers for the γ-emitter technetium tricarbonyl moiety. Inorg. Chim. Acta 2009, 362, 4785–4790. [Google Scholar] [CrossRef]
- Alberto, R.; Egli, A.; Abram, U.; Hegetschweiler, K.; Gramlich, V.; Schubiger, P.A. Synthesis and reactivity of [NEt4]2[ReBr3(CO)3]. Formation and structural characterization of the clusters [NEt4][Re3(µ3-OH)(µ-OH)3(CO)9] and [NEt4][Re2(µ-OH)3(CO)6] by alkaline titration. J. Chem. Soc. Dalton Trans. 1994, 2815–2820. [Google Scholar] [CrossRef]
- Cardoso, J.M.S.; Correia, I.; Galvao, A.M.; Marques, F.; Carvalho, M. Synthesis of Ag(I) camphor sulphonylimine complexes and assessment of their cytotoxic properties against cisplcitin-resistant A2780cisR and A2780 cell lines. J. Inorg. Biochem. 2017, 166, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ravera, M.; Gabano, E.; Zanellato, I.; Perin, E.; Arrais, A.; Osella, D. Polyanionic biopolymers for the delivery of Pt(II) cationic antiproliferative complexes. Bioinorg. Chem. Appl. 2016, 2016, 2380540. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.; Fanti, M.; Medda, L.; Nairi, V.; Cugia, F.; Piludu, M.; Sogos, V.; Monduzzi, M. Mesoporous silica nanoparticles functionalized with hyaluronic acid and chitosan biopolymers. Effect of functionalization on cell internalization. Acs Biomater. Sci. Eng. 2016, 2, 741–751. [Google Scholar] [CrossRef]
- Viola-Villegas, N.; Rabideau, A.E.; Cesnavicious, J.; Zubieta, J.; Doyle, R.P. Targeting the folate receptor (FR): Imaging and cytotoxicity of Re–I conjugates in FR-overexpressing cancer cells. ChemMedChem 2008, 3, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, A.; Aceto, M.; Cassino, C.; Gabano, E.; Osella, D. Uptake of antitumor platinum(II)-complexes by cancer cells, assayed by inductively coupled plasma mass spectrometry (ICP-MS). J. Inorg. Biochem. 2004, 98, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Gabano, E.; Colangelo, D.; Ghezzi, A.R.; Osella, D. The influence of temperature on antiproliferative effects, cellular uptake and DNA platination of the clinically employed Pt(II)-drugs. J. Inorg. Biochem. 2008, 102, 629–635. [Google Scholar] [CrossRef] [PubMed]




| Compound | ζ-potential (mV) | DLS Diameter (nm) |
|---|---|---|
| C1 | 41.5 ± 3.1 | 180 ± 10 |
| C12 | 37.5 ± 0.6 | 215 ± 12 |
| C123 | 45.9 ± 1.1 | 298 ± 21 |
| C1234 | 39.0 ± 2.4 | 300 ± 24 |
| Compound or Conjugate | IC50 (mg·mL−1) 1 | IC50 (µM) 2 | Pt AR | Re AR |
|---|---|---|---|---|
| cisplatin | - | 20.7 ± 5.6 | - | - |
| 2 | - | 13.7 ± 3.0 | 0.385 ± 0.091 3 | |
| C1 | 2.06 ± 0.83 | - | - | - |
| C12 | 1.34 ± 0.35 | 33.7 ± 8.9 | 3.56 ± 0.19 | - |
| C123 | 0.43 ± 0.06 | 13.5 ± 2.0 | - | - |
| C1234 | 0.65 ± 0.29 | 26.0 ± 11.8 | 2.31 ± 0.12 | 3.00 ± 0.87 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabano, E.; Do Quental, L.; Perin, E.; Silva, F.; Raposinho, P.; Paulo, A.; Ravera, M. Pt(IV)/Re(I) Chitosan Conjugates as a Flexible Platform for the Transport of Therapeutic and/or Diagnostic Anticancer Agents. Inorganics 2018, 6, 4. https://doi.org/10.3390/inorganics6010004
Gabano E, Do Quental L, Perin E, Silva F, Raposinho P, Paulo A, Ravera M. Pt(IV)/Re(I) Chitosan Conjugates as a Flexible Platform for the Transport of Therapeutic and/or Diagnostic Anticancer Agents. Inorganics. 2018; 6(1):4. https://doi.org/10.3390/inorganics6010004
Chicago/Turabian StyleGabano, Elisabetta, Letícia Do Quental, Elena Perin, Francisco Silva, Paula Raposinho, António Paulo, and Mauro Ravera. 2018. "Pt(IV)/Re(I) Chitosan Conjugates as a Flexible Platform for the Transport of Therapeutic and/or Diagnostic Anticancer Agents" Inorganics 6, no. 1: 4. https://doi.org/10.3390/inorganics6010004
APA StyleGabano, E., Do Quental, L., Perin, E., Silva, F., Raposinho, P., Paulo, A., & Ravera, M. (2018). Pt(IV)/Re(I) Chitosan Conjugates as a Flexible Platform for the Transport of Therapeutic and/or Diagnostic Anticancer Agents. Inorganics, 6(1), 4. https://doi.org/10.3390/inorganics6010004