[2 × 2] Molecular Grids of Ni(II) and Zn(II) with Redox-Active 1,4-Pyrazine-Bis(thiosemicarbazone) Ligands
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterisation
2.2. Properties of the Solids
2.3. UV-Vis Absorption Spectroscopy
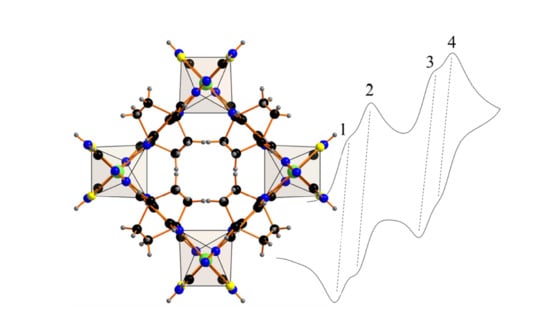
2.4. Electrochemistry
2.5. Spectroelectrochemical UV-Vis Absorption and EPR Spectroscopy
2.6. Quantumchemical Calculations
3. Experimental Section
3.1. Methods and Instrumentation
3.2. Syntheses
3.2.1. General
3.2.2. Synthesis of the tetranuclear complexes [M4(LR)4]
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jansson, P.J.; Kalinowski, D.S.; Lane, D.J.R.; Kovacevic, Z.; Seebacher, N.A.; Fouani, L.; Sahne, S.; Merlot, A.M.; Richardson, D.R. The renaissance of polypharmacology in the development of anti-cancer therapeutics: Inhibition of the “Triad of Death” in cancer by Di-2-pyridylketone thiosemicarbazones. Pharmacol. Res. 2015, 100, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.R.; Mills, T.M.; Shafie, N.H.; Merlot, A.M.; Moussa, R.S.; Kalinowski, D.S.; Kovacevic, Z.; Richardson, D.R. Expanding horizons in iron chelation and the treatment of cancer: Role of iron in the regulation of ER stress and the epithelial–mesenchymal transition. Biochim. Biophys. Acta 2014, 1845, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, J.R.; Hueting, R. Metal complexes of thiosemicarbazones for imaging and therapy. Inorg. Chim. Acta 2012, 389, 3–15. [Google Scholar] [CrossRef]
- Paterson, B.M.; Donnelly, P.S. Copper complexes of bis(thiosemicarbazones): From chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals. Chem. Soc. Rev. 2011, 40, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Ettari, R.; Bova, F.; Zappala, M.; Grasso, S.; Micale, N. Falcipain-2 Inhibitors. Med. Res. Rev. 2010, 30, 136–167. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G.; Bisceglie, F.; Bignami, F.; Ronzi, P.; Schiavone, P.; Re, M.C.; Casoli, C.; Pilotti, E. Antiretroviral Activity of Thiosemicarbazone Metal Complexes. J. Med. Chem. 2010, 53, 8765–8769. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G. Thiosemicarbazone Metal Complexes: From Structure to Activity. Open Cryst. J. 2010, 3, 16–28. [Google Scholar] [CrossRef]
- Lobana, T.S.; Sharma, R.; Bawa, G.; Khanna, S. Bonding and structure trends of thiosemicarbazone derivatives of metals‒An overview. Coord. Chem. Rev. 2009, 253, 977–1055. [Google Scholar] [CrossRef]
- Christlieb, M.; Dilworth, J.R. Ligands for Molecular Imaging: The Synthesis of Bis(thiosemicarbazone) Ligands. Chem.-Eur. J. 2006, 12, 6194–6206. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, A.G.; Ranninger, C.N. Contribution to the SAR field of metallated and coordination complexes. Studies of the palladium and platinum derivatives with selected thiosemicarbazones as antitumoral drugs. Coord. Chem. Rev. 2004, 248, 118–133. [Google Scholar] [CrossRef]
- Baartzes, N.; Stringer, T.; Okombo, J.; Seldon, R.; Warner, D.F.; de Kock, C.; Smith, P.J.; Smith, G.S. Mono- and polynuclear ferrocenylthiosemicarbazones: Synthesis, characterisation and antimicrobial evaluation. J. Organomet. Chem. 2016, 819, 166–172. [Google Scholar] [CrossRef]
- Indoria, S.; Lobana, T.S.; Singh, D.; Kumari, S.; Kumari, P.; Bala, T.; Kamal, A.; Jassal, A.K.; García Santos, I.; Castineiras, A.; et al. Stabilization of CuII–I Bonds Using 2-Benzoylpyridine Thiosemicarbazones—Synthesis, Structure, Spectroscopy, Fluorescence, and Cyclic Voltammetry. Eur. J. Inorg. Chem. 2015, 2015, 5106–5117. [Google Scholar] [CrossRef]
- Qi, J.; Liang, S.; Gou, Y.; Zhang, Z.; Zhou, Z.; Yang, F.; Liang, H. Synthesis of four binuclear copper(II) complexes: Structure, anticancer properties and anticancer mechanism. Eur. J. Med. Chem. 2015, 96, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Bao, W.-G.; Tian, H.; Li, B.; Zhao, X.-F.; Qiao, X.; Xu, J.-Y. Nuclease activity and protein-binding properties of a novel tetranuclear thiosemicarbazide Pt(II) complex. Dalton Trans. 2014, 43, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.T.; Antelo, J.M.; Adrio, L.A.; Martinez, J.; Ortigueira, J.M.; Lopez-Torres, M.; Vila, J.M. Novel Bidentate [N,S] Palladacycle Metalloligands. 1H−15N HMBC as a Decisive NMR Technique for the Structural Characterization of Palladium−Rhodium and Palladium−Palladium Bimetallic Complexes. Organometallics 2014, 33, 3265–3274. [Google Scholar] [CrossRef]
- Adams, M.; de Kock, C.; Smith, P.J.; Chibale, K.; Smith, G.S. Synthesis, characterization and antiplasmodial evaluation of cyclopalladated thiosemicarbazone complexes. J. Organomet. Chem. 2013, 736, 19–26. [Google Scholar] [CrossRef]
- Demoro, B.; de Almeida, R.F.M.; Marques, F.; Matos, C.P.; Otero, L.; Pessoa, J.C.; Santos, I.; Rodríguez, A.; Moreno, V.; Lorenzo, J.; et al. Screening organometallic binuclear thiosemicarbazone ruthenium complexes as potential anti-tumour agents: Cytotoxic activity and human serum albumin binding mechanism. Dalton Trans. 2013, 42, 7131–7146. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Nimir, H.; Aktas, C.; Huch, V.; Rauch, U.; Schäfer, K.-H.; Veith, M. Organoplatinum(II) Complexes with 2-Acetylthiophene Thiosemicarbazone: Synthesis, Characterization, Crystal Structures, and in Vitro Antitumor Activity. Organometallics 2012, 31, 2256–2262. [Google Scholar] [CrossRef]
- Chellan, P.; Land, K.M.; Shokar, A.; Au, A.; An, S.H.; Clavel, C.M.; Dyson, P.J.; de Kock, C.; Smith, P.J.; Chibale, K.; et al. Exploring the Versatility of Cycloplatinated Thiosemicarbazones as Antitumor and Antiparasitic Agents. Organometallics 2012, 31, 5791–5799. [Google Scholar] [CrossRef]
- Latheef, L.; Seena, E.B.; Kurup, M.R.P. Synthesis, spectral and structural studies of novel binuclear Ni(II) complex of salicylaldehyde 3-azacyclothiosemicarbazone. Inorg. Chim. Acta 2009, 362, 2515–2518. [Google Scholar] [CrossRef]
- Philip, V.; Suni, V.; Kurup, M.R.P.; Nethaji, M. Copper(II) complexes derived from di-2-pyridyl ketone N(4),N(4)-(butane-1,4-diyl)thiosemicarbazone: Crystal structure and spectral studies. Polyhedron 2006, 25, 1931–1938. [Google Scholar] [CrossRef]
- Panja, A.; Campana, C.; Leavitt, C.; Van Stipdonk, M.J.; Eichhorn, D.M. Iron and cobalt complexes of 2,6-diacetylpyridine-bis(R-thiosemicarbazone) (R = H, phenyl) showing unprecedented ligand deviation from planarity. Inorg. Chim. Acta 2009, 362, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Pedrido, R.; Gonzalez-Noya, A.M.; Romero, M.J.; Martinez-Calvo, M.; Vazquez Lopez, M.; Gomez-Forneas, E.; Zaragoza, G.; Bermejo, M.R. Pentadentate thiosemicarbazones as versatile chelating systems. A comparative structural study of their metallic complexes. Dalton Trans. 2008, 47, 6776–6787. [Google Scholar] [CrossRef] [PubMed]
- Matsinha, L.C.; Mao, J.; Mapolie, S.F.; Smith, G.S. Water-Soluble Palladium(II) Sulfonated Thiosemicarbazone Complexes: Facile Synthesis and Preliminary Catalytic Studies in the Suzuki–Miyaura Cross-Coupling Reaction in Water. Eur. J. Inorg. Chem. 2015, 2015, 4088–4094. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Tapia, S.; Souza, P. First 3,5-diacetyl-1,2,4-triazol derived mono(thiosemicarbazone) and its palladium and platinum complexes: Synthesis, structure and biological properties. Inorg. Chim. Acta 2016, 445, 62–69. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Perles, J.; Souza, P. New palladium and platinum complexes with bioactive 3,5-diacetyl-1,2,4-triazol bis(4-cyclohexyl thiosemicarbazone) ligand: Chemistry, antiproliferative activity and preliminary toxicity studies. Dalton Trans. 2012, 41, 12538–12547. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, A.I.; Pastor, C.; Souza, P. Synthesis and structural characterization of a disulphide-bridged tetranuclear palladium(II) complex derived from 3,5-diacetyl 1,2,4-triazole bis(4-ethylthiosemicarbazone). Inorg. Chem. Commun. 2007, 10, 97–100. [Google Scholar] [CrossRef]
- Kasuga, N.C.; Sekino, K.; Koumo, C.; Shimada, N.; Ishikawa, M.; Nomiya, K. Synthesis, structural characterization and antimicrobial activities of 4- and 6-coordinate nickel(II) complexes with three thiosemicarbazones and semicarbazone ligands. J. Inorg. Biochem. 2001, 84, 55–65. [Google Scholar] [CrossRef]
- De Sousa, G.F.; West, D.X.; Brown, C.A.; Swearingen, J.K.; Valdes-Martinez, J.; Toscano, R.A.; Hernandez-Ortega, S.; Hörner, M.; Bortoluzzi, A.J. Structural and spectral studies of a heterocyclic N(4)-substituted bis(thiosemicarbazone), H22,6Achexim·H2O, its heptacoordinated tin(IV) complex [Bu2Sn(2,6Achexim)], and its binuclear zinc(II) complex [Zn(2,6Achexim)]2. Polyhedron 2000, 19, 841–847. [Google Scholar] [CrossRef]
- Kulkarni, N.V.; Revankar, V.K. Synthesis, antimicrobial screening, and DNA-binding/cleavage of new pyrazole-based binuclear CoII, NiII, CuII, and ZnII complexes. J. Coord. Chem. 2011, 64, 725–741. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Hernandez, C.; Rodriguez, A.; Souza, P. Novel bis(thiosemicarbazones) of the 3,5-diacetyl-1,2,4-triazol series and their platinum(II) complexes: Chemistry, antiproliferative activity and preliminary nephrotoxicity studies. Dalton Trans. 2011, 40, 5738–5745. [Google Scholar] [CrossRef] [PubMed]
- Drover, M.W.; Tandon, S.S.; Anwar, M.U.; Shuvaev, K.V.; Dawe, L.N.; Collins, J.L.; Thompson, L.K. Polynuclear complexes of a series of hydrazone and hydrazone–oxime ligands—M2 (Fe), M4 (Mn, Ni, Cu), and Mn (Cu) examples. Polyhedron 2014, 68, 94–102. [Google Scholar] [CrossRef]
- Kowol, C.R.; Reisner, E.; Chiorescu, I.; Arion, V.B.; Galanski, M.; Deubel, D.V.; Keppler, B.K. An Electrochemical Study of Antineoplastic Gallium, Iron and Ruthenium Complexes with Redox Noninnocent α-N-Heterocyclic Chalcogensemicarbazones. Inorg. Chem. 2008, 47, 11032–11047. [Google Scholar] [CrossRef] [PubMed]
- Kowol, C.R.; Berger, R.; Eichinger, R.; Roller, A.; Jakupec, M.A.; Schmidt, P.P.; Arion, V.B.; Keppler, B.K. Gallium(III) and Iron(III) Complexes of α-N-Heterocyclic Thiosemicarbazones: Synthesis, Characterization, Cytotoxicity, and Interaction with Ribonucleotide Reductase. J. Med. Chem. 2007, 50, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-X.; Zhang, L.-Z.; Yang, M.; Niu, J.-Y.; Zhou, J. Synthesis, crystal structures, in vitro biological evaluation of zinc(II) and bismuth(III) complexes of 2-acetylpyrazine N(4)-phenylthiosemicarbazone. Bioorg. Med. Chem. Lett. 2012, 22, 2418–2423. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Divilov, V.; Lewis, J.S. Role of Metalation in the Topoisomerase IIα Inhibition and Antiproliferation Activity of a Series of α-Heterocyclic-N4-Substituted Thiosemicarbazones and Their Cu(II) Complexes. J. Med. Chem. 2011, 54, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, Q.; Bai, Y.; Duan, C.; Zhang, B.; Meng, Q. Chiral aggregation and spontaneous resolution of thiosemicarbazone metal complexes. Dalton Trans. 2006, 2572–2578. [Google Scholar] [CrossRef] [PubMed]
- West, D.X.; Bain, G.A.; Butcher, R.J.; Jasinski, J.P.; Pozdniakiv, R.Y.; Valdes-Martinez, J.; Toscano, R.A.; Hernandez-Ortega, S. Structural Studies of three Isomeric Forms of Heterocyclic N(4)-Substituted Thiosemicarbazones and two Nickel(II) Complexes. Polyhedron 1996, 15, 665–674. [Google Scholar] [CrossRef]
- Kaim, W.; Sarkar, B.; Lahiri, G.K. Mixed Valence Intermediates as Ideal Targets für Spectroelectrochemistry in Spectroelectrochemistry; Kaim, W., Klein, A., Eds.; RSC Publishing: Cambridge, UK, 2008; Chapter 3; pp. 68–90. ISBN 978-0-85404-550-1. [Google Scholar] [CrossRef]
- Kaim, W.; Klein, A.; Glöckle, M. Exploration of Mixed-Valence Chemistry: Inventing New Analogues of the Creutz-Taube Ion. Acc. Chem. Res. 2000, 33, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Kaim, W.; Fiedler, J. Spectroelectrochemistry: The best of two worlds. Chem. Soc. Rev. 2009, 38, 3373–3382. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G. Metallosupramolecular grid complexes: Towards nanostructured materials with high-tech applications. Chem. Soc. Rev. 2013, 42, 7881–7899. [Google Scholar] [CrossRef] [PubMed]
- Dawe, L.N.; Shuvaev, K.V.; Thompson, L.K. Polytopic ligand directed self-assembly—Polymetallic [n × n] grids versus non-grid oligomers. Chem. Soc. Rev. 2009, 38, 2334–2359. [Google Scholar] [CrossRef] [PubMed]
- Stefankiewicz, A.R.; Lehn, J.-M. Highly Sensitive Magnetic Effects Induced by Hydrogen-Bonding Interactions in a High-Spin Metallosupramolecular Fe4II [2 × 2] Grid-Type Complex. Chem. Eur. J. 2009, 15, 2500–2503. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Huang, W.; Wu, D.; Zheng, Z.; Huang, X.-C.; Sato, O. Redox Modulation of Spin Crossover within a Cobalt Metallogrid. Inorg. Chem. 2016, 55, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Q.; Wang, Y.-T.; Cui, A.-I.; Kou, H.-Z. Toward Higher Nuclearity: Tetranuclear Cobalt(II) Metallogrid Exhibiting Spin Crossover. Inorg. Chem. 2014, 53, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Li, S.-T.; Wu, S.-Q.; Cui, A.-L.; Shen, D.-Z.; Kou, H.-Z. Spin Transitions in Fe(II) Metallogrids Modulated by Substituents, Counteranions, and Solvents. J. Am. Chem. Soc. 2013, 135, 5942–5945. [Google Scholar] [CrossRef] [PubMed]
- Steinert, M.; Schneider, B.; Dechert, S.; Demeshko, S.; Meyer, F. Spin-State Versatility in a Series of Fe4 [2 × 2] Grid Complexes: Effects of Counteranions, Lattice Solvent, and Intramolecular Cooperativity. Inorg. Chem. 2016, 55, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Demeshko, S.; John, M.; Dechert, S.; Meyer, F. Redox-Induced Single-Molecule Magnetism in Mixed-Valent [2 × 2] Co4 Grid Complexes. Inorg. Chem. 2016, 55, 4362–4372. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Demeshko, S.; Neudeck, S.; Dechert, S.; Meyer, F. Mixed-Spin [2 × 2] Fe4 Grid Complex Optimized for Quantum Cellular Automata. Inorg. Chem. 2013, 52, 13230–13237. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cao, F.; Li, D.; Zeng, S.; Song, Y.; Dou, J. Solvent dependent reactivities of di-, tetra- and hexanuclear manganese complexes: Syntheses, structures and magnetic properties. Dalton Trans. 2015, 44, 6620–6629. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Newton, G.N.; Shiga, T.; Hayami, S.; Matsui, Y.; Okamoto, H.; Kumai, R.; Murakami, Y.; Oshio, H. Programmable spin-state switching in a mixed-valence spin-crossover iron grid. Nat. Commun. 2014, 5, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, B.; Wang, R.; Zhang, H.; Niu, C.; Niu, Y.; Hou, H. Hierarchical Assembly of Extended Coordination Networks Constructed by Novel Metallacalix[4]arenes Building Blocks. Inorg. Chem. 2010, 49, 2600–2613. [Google Scholar] [CrossRef] [PubMed]
- Campos-Fernandez, C.S.; Schottel, B.L.; Chifotides, H.T.; Bera, J.K.; Bacsa, J.; Koomen, J.M.; Russell, D.H.; Dunbar, K.R. Anion Template Effect on the Self-Assembly and Interconversion of Metallacyclophanes. J. Am. Chem. Soc. 2005, 127, 12909–12923. [Google Scholar] [CrossRef] [PubMed]
- Campos-Fernandez, C.S.; Clerac, R.; Dunbar, K.R. A One-Pot, High-Yield Synthesis of a Paramagnetic Nickel Square from Divergent Precursors by Anion Template Assembly. Angew. Chem. Int. Ed. 1999, 38, 3477–3479. [Google Scholar] [CrossRef]
- Li, X.-L.; Kang, J.-L.; Zhang, X.-L.; Xiao, H.-P.; Wang, A.-L.; Zhou, L.; Fang, S.-M.; Liu, C.-M. Anion-controlled self-assembly of two NLO-active dinuclear and molecular square Cu(II) enantiomeric pairs: From antiferromagnetic to ferromagnetic coupling. Dalton Trans. 2014, 43, 17226–17229. [Google Scholar] [CrossRef] [PubMed]
- Bark, T.; Düggeli, M.; Stoeckli-Evans, H.; von Zelewsky, A. Designed Molecules for Self-Assembly: The Controlled Formation of Two Chiral Self-Assembled Polynuclear Species with Predetermined Configuration. Angew. Chem. Int. Ed. 2001, 40, 2848–2851. [Google Scholar] [CrossRef]
- Pace, G.; Stefankiewicz, A.; Harrowfield, J.; Lehn, J.-M.; Samor, P. Self-Assembly of Alkoxy-Substituted Bis(hydrazone)-Based Organic Ligands and of a Metallosupramolecular Grid on Graphite. Chem. Phys. Chem. 2009, 10, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Ruben, M.; Lehn, J.-M.; Müller, P. Addressing metal centres in supramolecular assemblies. Chem. Soc. Rev. 2006, 35, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Caronna, T.; Fronza, G.; Minisci, F.; Porta, O. Homolytic Acylation of Protonated Pyridine and Pyrazine Derivatives. J. Chem. Soc. Perkin Trans. 2 1972, 2035–3038. [Google Scholar] [CrossRef]
- Moroz, Y.S.; Demeshko, S.; Haukka, M.; Mokhir, A.; Mitra, U.; Stocker, M.; Müller, P.; Meyer, F.; Fritsky, I.O. Regular High-Nuclearity Species from Square Building Blocks: A Triangular 3 × [2 × 2] Ni12 Complex Generated by the Self-assembly of Three [2 × 2] Ni4 Molecular Grids. Inorg. Chem. 2012, 51, 7445–7447. [Google Scholar] [CrossRef] [PubMed]
- Sachse, A.; Demeshko, S.; Dechert, S.; Daebel, V.; Lange, A.; Meyer, F. Highly preorganized pyrazolate-bridged palladium(II) and nickel(II) complexes in bimetallic norbornene polymerization. Dalton Trans. 2010, 39, 3903–3914. [Google Scholar] [CrossRef] [PubMed]
- Moroz, Y.S.; Kulon, K.; Haukka, M.; Gumienna-Kontecka, E.; Kozłowski, H.; Meyer, F.; Fritsky, I.O. Synthesis and Structure of [2 × 2] Molecular Grid Copper(II) and Nickel(II) Complexes with a New Polydentate Oxime-Containing Schiff Base Ligand. Inorg. Chem. 2008, 47, 5656–5665. [Google Scholar] [CrossRef] [PubMed]
- Klingele, J.; Prikhod’ko, A.I.; Leibeling, G.; Demeshko, S.; Dechert, S.; Meyer, F. Pyrazolate-based copper(II) and nickel(II) [2 × 2] grid complexes: Protonation-dependent self-assembly, structures and properties. Dalton Trans. 2007, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-Y.; Harrowfield, J.; Nitschke, J.; Ramírez, J.; Stadler, A.-M.; Kyritsakas-Gruber, N.; Madalan, A.; Rissanen, K.; Russo, L.; Vaughan, G.; et al. Generation of [2 × 2] Grid Metallosupramolecular Architectures from Preformed Ditopic Bis(acylhydrazone) Ligands and through Component Self-Assembly. Eur. J. Inorg. Chem. 2007, 2007, 2944–2965. [Google Scholar] [CrossRef]
- Dinolfo, P.H.; Hupp, J.T. Tetra-Rhenium Molecular Rectangles as Organizational Motifs for the Investigation of Ligand-Centered Mixed Valency: Three Examples of Full Delocalization. J. Am. Chem. Soc. 2004, 126, 16814–16819. [Google Scholar] [CrossRef] [PubMed]
- Dinolfo, P.H.; Williams, M.E.; Stern, C.L.; Hupp, J.T. Rhenium-Based Molecular Rectangles as Frameworks for Ligand-Centered Mixed Valency and Optical Electron Transfer. J. Am. Chem. Soc. 2004, 126, 12989–13001. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Thanasekaran, P.; Cheng, Y.-W.; Lee, C.-C.; Manimaran, B.; Rajendran, T.; Liao, R.-T.; Lee, G.-H.; Peng, S.-M.; Lu, K.-L. Unprecedented Reduction of 2,2′-Bipyrimidine in a One-Pot Synthesis of Neutral Rhenium(I)-Based Molecular Rectangles. Organometallics 2008, 27, 2141–2144. [Google Scholar] [CrossRef]
- Dinolfo, P.H.; Lee, S.J.; Coropceanu, V.; Bredas, J.-L.; Hupp, J.T. Borderline Class II/III Ligand-Centered Mixed Valency in a Porphyrinic Molecular Rectangle. Inorg. Chem. 2005, 44, 5789–5797. [Google Scholar] [CrossRef] [PubMed]
- Low, P.J.; Brown, N.J. Electronic Interactions Between and Through Covalently-Bonded Polymetallic Complexes. J. Clust. Sci. 2010, 21, 235–278. [Google Scholar] [CrossRef]
- Mücke, P.; Winter, R.F.; Novak, I.; Kowalski, K. Synthesis, spectroelectrochemistry and electronic structure calculations of 4-(2-ferrocenylvinyl)-[2.2]-paracyclophane and 4,12-di-(2-ferrocenylvinyl)-[2.2]-paracyclophane. J. Organomet. Chem. 2012, 717, 14–22. [Google Scholar] [CrossRef]
- Safin, D.A.; Babashkina, M.G.; Bolte, M.; Mitoraj, M.P.; Klein, A. Metal ion influences distortion of the ligand in the structure of [M{2-MeO(O)CC6H4NHC(S)NP(S)(OiPr)2}2] (M = ZnII, CdII) complexes: A driving force for the intermolecular aggregation. Dalton Trans. 2015, 44, 14101–14109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Mazzone, A.; Polidori, G.; Spagna, R. Sir-2011, A Program for Automatic Solution and Refinement of Crystal Structures; CNR Institute of Crystallography: Bari, Italy, 2012. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. Sir-92, A Program for Automatic Solution and Refinement of Crystal Structures; CNR Institute of Crystallography: Bari, Italy, 1994. [Google Scholar]
- Sheldrick, G.M. SHELXL-2013. Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 2008. [Google Scholar]
- Sheldrick, G.M. SHELXL-97. Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 2008. [Google Scholar]
- X-Red 1.07; Stoe &Cie GmbH: Darmstadt, Germany, 1996.
- X-Shape 1.0.1; Stoe &Cie GmbH: Darmstadt, Germany, 1996.
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON, A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 1998. [Google Scholar]
- Waltersperger, S.; Olieric, V.; Pradervand, C.; Glettig, W.; Salathe, M.; Fuchs, M.R.; Curtin, A.; Wang, X.; Ebner, S.; Panepucci, E.; et al. PRIGo: A new multi-axis goniometer for macromolecular crystallography. J. Synchrotron Radiat. 2015, 22, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- TURBOMOLE V7.0 2015, a Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH. 2007. Available online: http://www.turbomole.com (accessed on 15 June 2015).
- Steffen, C.; Thomas, K.; Huniar, U.; Hellweg, A.; Rubner, O.; Schroer, A. TmoleX—A Graphical User Interface for TURBOMOLE. J. Comput. Chem. 2010, 31, 2967–2970. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]











| Epa ox1 | E1/2 red1 | Epc red2 | Epc red3 | Epc red4 | |||
|---|---|---|---|---|---|---|---|
| H2LMe | - | - | - | −1.69 | −2.05 | −2.62 | −2.81 |
| H2LEt | - | - | 0.62 | −1.67 | −2.13 | −2.75 | −3.25 |
| H2LiPr | - | - | 0.60 | −1.63 | −2.11 | −2.51 | −2.66 |
| H2LPh | - | - | 0.50 | −1.48 | −1.70 | −1.97 | −2.45 |
| Epa ox3 | Epa ox2 | Epa ox1 | E1/2 red1 | E1/2 red2 | E1/2 red3 | E1/2 red4 | |
| [Zn4(LiPr)4] | - | 0.80 | 0.55 | −1.54 | −1.65 | −1.93 | −2.03 |
| [Zn4(LPh)4] | - | 0.79 | 0.53 | −1.41 | −1.52 | −1.86 | −1.91 |
| [Ni4(LEt)4] | - | 0.21 | 0.04 | −1.47 | −1.56 | −1.82 | −1.95 |
| [Ni4(LiPr)4] | 0.72 | 0.21 | 0.02 | −1.59 | −1.71 | −2.02 | −2.13 |
| [Ni4(LPh)4] | 0.80 | 0.32 | 0.29 | −1.43 | −1.54 | −1.82 | −1.94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arefyeva, N.; Sandleben, A.; Krest, A.; Baumann, U.; Schäfer, M.; Kempf, M.; Klein, A. [2 × 2] Molecular Grids of Ni(II) and Zn(II) with Redox-Active 1,4-Pyrazine-Bis(thiosemicarbazone) Ligands. Inorganics 2018, 6, 51. https://doi.org/10.3390/inorganics6020051
Arefyeva N, Sandleben A, Krest A, Baumann U, Schäfer M, Kempf M, Klein A. [2 × 2] Molecular Grids of Ni(II) and Zn(II) with Redox-Active 1,4-Pyrazine-Bis(thiosemicarbazone) Ligands. Inorganics. 2018; 6(2):51. https://doi.org/10.3390/inorganics6020051
Chicago/Turabian StyleArefyeva, Natalia, Aaron Sandleben, Alexander Krest, Ulrich Baumann, Mathias Schäfer, Maxim Kempf, and Axel Klein. 2018. "[2 × 2] Molecular Grids of Ni(II) and Zn(II) with Redox-Active 1,4-Pyrazine-Bis(thiosemicarbazone) Ligands" Inorganics 6, no. 2: 51. https://doi.org/10.3390/inorganics6020051
APA StyleArefyeva, N., Sandleben, A., Krest, A., Baumann, U., Schäfer, M., Kempf, M., & Klein, A. (2018). [2 × 2] Molecular Grids of Ni(II) and Zn(II) with Redox-Active 1,4-Pyrazine-Bis(thiosemicarbazone) Ligands. Inorganics, 6(2), 51. https://doi.org/10.3390/inorganics6020051