Synthesis of Ferrocenyl-Substituted Organochalcogenyldichlorogermanes
Abstract
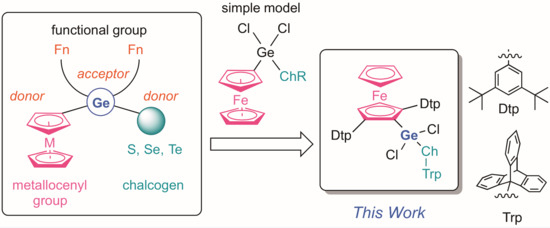
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. General Information
3.2. Experimental Details
3.2.1. Synthesis of Selenenylchloride 2a
3.2.2. Synthesis of Tellurenylchloride 2b
3.2.3. Reaction of Chlorogermylenoid 4 with Selenenylchloride 2a
3.2.4. Reaction of Chlorogermylenoid 4 with Tellurenylchloride 2b
3.3. Computational Methods
3.4. X-ray Crystallographic Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vaughn, D.D., II; Schaak, R.E. Synthesis, properties and applications of colloidal germanium and germanium-based nanomaterials. Chem. Soc. Rev. 2013, 42, 2861–2879. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Jung, E.A.; Han, S.H.; Han, J.H.; Park, B.K.; Kim, C.G.; Chung, T.-M. Germanium Compounds Containing Ge=E Double Bonds (E = S, Se, Te) as Single-Source Precursors for Germanium Chalcogenide Materials. Inorg. Chem. 2017, 56, 4084–4092. [Google Scholar] [CrossRef] [PubMed]
- Sasamori, T.; Sakagami, M.; Niwa, M.; Sakai, H.; Furukawa, Y.; Tokitoh, N. Synthesis of a stable 1,2-bis(ferrocenyl)diphosphene. Chem. Commun. 2012, 48, 8562–8564. [Google Scholar] [CrossRef] [PubMed]
- Sasamori, T.; Suzuki, Y.; Sakagami, M.; Miyake, H.; Tokitoh, N. Structure of stable telluradiphosphirane bearing bulky ferrocenyl ligands. Chem. Lett. 2014, 43, 1464–1466. [Google Scholar] [CrossRef]
- Sasamori, T.; Suzuki, Y.; Tokitoh, N. Isolation and Structural Characterization of a Lewis Base-Free Monolithioferrocene. Organometallics 2014, 33, 6696–6699. [Google Scholar] [CrossRef]
- Sakagami, M.; Sasamori, T.; Sakai, H.; Furukawa, Y.; Tokitoh, N. 1,2-Bis(ferrocenyl)dipnictenes: Bimetallic Systems with a Pn = Pn Heavy π-Spacer (Pn: P, Sb, and Bi). Bull. Chem. Soc. Jpn. 2013, 86, 1132–1143. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sasamori, T.; Guo, J.-D.; Nagase, S.; Tokitoh, N. Isolation and Ambident Reactivity of a Chlorogermylenoid. Chem. Eur. J. 2016, 22, 13784–13788. [Google Scholar] [CrossRef] [PubMed]
- Sasamori, T.; Sugamata, K.; Tokitoh, N. Halogenation reactions of a ditelluride having bulky aryl groups leading to the formation of organotellurium halides. Heteroat. Chem. 2011, 22, 405–411. [Google Scholar] [CrossRef]
- Beckmann, J.; Hesse, M.; Poleschner, H.; Seppelt, K. Formation of mixed-valent aryltellurenyl halides RX2TeTeR. Angew. Chem. Int. Ed. 2007, 46, 8277–8280. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, J.; Heitz, S.; Hesse, M. Four distinctively different decomposition pathways of metastable supermesityltellurium (IV) trichloride. Inorg. Chem. 2007, 46, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Matsubayashi, S.; Takahashi, T.; Nakayama, J. Preparation of a Selenenic Acid and Isolation of Selenoseleninates. J. Org. Chem. 1999, 64, 1084–1085. [Google Scholar] [CrossRef]
- Baker, R.J.; Jones, C. The molecular structure of ditriptycenyl ditelluride. Main Group Met. Chem. 2004, 27, 323–325. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nakata, N.; Ishii, A. Strong Solid-State Phosphorescence of 1,2-Telluraplatinacycles Incorporated into Rigid Dibenzobarrelene and Triptycene Skeletons. Eur. J. Inorg. Chem. 2013, 2013, 5233–5239. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sasamori, T.; Guo, J.-D.; Tokitoh, N. A Redox-Active Bis(ferrocenyl)germylene and Its Reactivity. Chem. Eur. J. 2018, 24, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scurseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, A.; et al. Gaussian09, revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural bond orbital analysis program. J. Comp. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Pangborn, A.B.; Giardello, M.A.; Grubbs, R.H.; Rosen, R.K.; Timmers, F.J. Safe and Convenient Procedure for Solvent Purification. Organometallics 2004, 15, 1518–1520. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]






| Distance/Å | 5a (Ch = Se) Form A Observed | 5a (Ch = Se) Form A Calculated | 5a (Ch = Se) Form B Calculated | 5b (Ch = Te) Form A Calculated | 5b (Ch = Te) Form B Calculated | 5b (Ch = Te) Form B Observed |
|---|---|---|---|---|---|---|
| C(Cp)–Ge | 1.926(6) | 1.9289 | 1.9290 | 1.9308 | 1.9309 | 1.927(8) |
| Ge–Ch | 2.3474(9) | 2.3870 | 2.3904 | 2.5878 | 2.5869 | 2.5489(8) |
| Ge–Cl1 | 2.209(2) | 2.1873 | 2.2018 | 2.1979 | 2.2081 | 2.212(2) |
| Ge–Cl2 | 2.164(2) | 2.1577 | 2.1561 | 2.1609 | 2.1624 | 2.164(2) |
| Fe···Ge | 3.696(1) | 3.6587 | 3.5460 | 3.7326 | 3.5252 | 3.505(2) |
| Fe···Cl1 | 4.194(2) | 4.0287 | 5.3396 | 4.2108 | 5.3303 | 5.305(3) |
| Fe···Cl2 | 5.176(2) | 5.1614 | 4.3337 | 5.1220 | 4.2763 | 4.317(2) |
| Fe···Ch | 5.229(1) | 5.3217 | 4.6049 | 5.6007 | 4.7724 | 4.761(1) |
| Angles/° | ||||||
| Cl1–Ge–Cl2 | 104.06(7) | 105.85 | 105.68 | 102.93 | 105.82 | 104.51(8) |
| Cl1–Ge–Ch | 110.59(5) | 103.47 | 108.33 | 108.95 | 108.17 | 106.62(6) |
| Cl2–Ge–Ch | 110.92(5) | 110.60 | 110.15 | 110.48 | 109.03 | 110.32(6) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasamori, T.; Suzuki, Y.; Sugamata, K.; Sugahara, T.; Tokitoh, N. Synthesis of Ferrocenyl-Substituted Organochalcogenyldichlorogermanes. Inorganics 2018, 6, 68. https://doi.org/10.3390/inorganics6030068
Sasamori T, Suzuki Y, Sugamata K, Sugahara T, Tokitoh N. Synthesis of Ferrocenyl-Substituted Organochalcogenyldichlorogermanes. Inorganics. 2018; 6(3):68. https://doi.org/10.3390/inorganics6030068
Chicago/Turabian StyleSasamori, Takahiro, Yuko Suzuki, Koh Sugamata, Tomohiro Sugahara, and Norihiro Tokitoh. 2018. "Synthesis of Ferrocenyl-Substituted Organochalcogenyldichlorogermanes" Inorganics 6, no. 3: 68. https://doi.org/10.3390/inorganics6030068
APA StyleSasamori, T., Suzuki, Y., Sugamata, K., Sugahara, T., & Tokitoh, N. (2018). Synthesis of Ferrocenyl-Substituted Organochalcogenyldichlorogermanes. Inorganics, 6(3), 68. https://doi.org/10.3390/inorganics6030068