Chiral Tectonics: VCD and ECD Application for Epimerization of a Star-Burst Tetranuclear Complex with a Labile Central Core
Abstract
:1. Introduction
2. Results
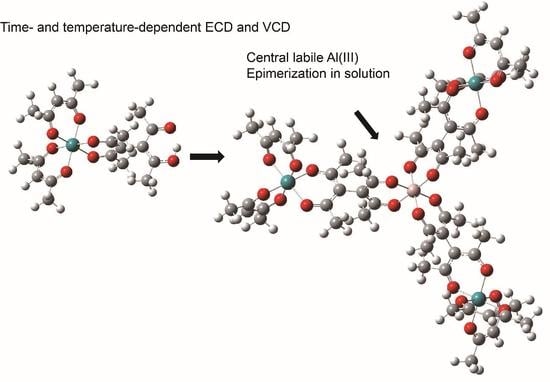
2.1. Preparation of a Star-Burst Tetranuclear Complex
2.2. Time-Dependent ECD Spectra
2.3. Epimerization Rate Cstants Using Temperature-Dependent ECD
2.4. VCD Application for the Epimerization
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amouri, H.; Gruselle, M. Chirality in Transion Metal Chemsitry; Wiley: West Sussex, UK, 2008; pp. 121–232. [Google Scholar]
- Riehl, J.P.; Kaizaki, S. Absolute Chiral Structures of Inorganic Compounds. In Physical Inorganic Chemistry; Bakac, A., Ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 143–197. [Google Scholar]
- Brock, A.J.; Clegg, J.K.; Li, F.; Lindoy, L.F. Recent developments in the metallo-supramolecular chemistry of oligo-β-diketonato ligands. Coord. Chem. Rev. 2018. [Google Scholar] [CrossRef]
- Jędrzejewska, H.; Szumna, A. Making a Right or Left Choice: Chiral Self-Sorting as a Tool for the Formation of Discrete Complex Structures. Chem. Rev. 2017, 117, 4863–4899. [Google Scholar] [CrossRef]
- Sato, H.; Yamagishi, A. Application of the ∆Λ Isomerism of Octahedral Metal Complexes as a Chiral Source in Photochemistry. J. Photochem. Photobiol. C Photochem. Rev. 2007, 8, 67–84. [Google Scholar] [CrossRef]
- Kaizaki, S. Applications of Electronic Circular Dichroism to Inorganic Sterochemsitry. In Comprehensive Chiroptical Spectroscopy; Berova, N., Polavarapu, P.L., Nakanishi, K., Woody, R.W., Eds.; Wiley: Hoboken, NJ, USA, 2012; Volume 2, pp. 451–471. [Google Scholar]
- Park, H.; Kim, K.Y.; Jung, S.H.; Choi, Y.; Sato, H.; Jung, J.H. Different Origins of Strain-Induced Chirality Inversion of Co2+-Triggered Supramolecular Peptide Polymers. Chem. Mater. 2018, 30, 2074–2083. [Google Scholar] [CrossRef]
- Xu, C.; Guenet, A.; Kyritsakas, N.; Planex, J.-M.; Hosseini, M.W. Molecular Tectonics: Design of Enantiopure Luminescent Heterometallic Ir(III)−Cd(II) Coordination Network. Inorg. Chem. 2015, 54, 10429–10439. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Yamagishi, A. VCD Studies on Chiral Characters of Metal Complex Oligomers. Int. J. Mol. Sci. 2013, 14, 964–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Furuno, Y.; Fukuda, Y.; Okamoto, K.; Yamagishi, A. Crystallization Induced Chiral Locking of a Central Labile Core in a Star-shaped Tetra-nuclear Complex. Dalton Trans. 2008, 10, 1283–1285. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Nakao, A.; Yamagishi, A. Formation of Chiral Heterometallic Oligomers by Combination of Inert Ru(III) Tectons and Labile Ni(II) Connectors. New J. Chem. 2011, 35, 1823–1828. [Google Scholar] [CrossRef]
- Fujimoto, N.; Mori, Y.; Yamagishi, A.; Sato, H. Molecular Recognition of Star-burst Tetranuclear Ru(III) Complexes on a Chirally Modified Clay Surface. Chem. Commun. 2010, 46, 5473–5475. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Sato, F.; Taniguchi, M.; Yamagishi, A. Chirality Effects on Core-periphery Connection in a Star-burst Type Tetranuclear Ru(III) Complex: Application of Vibrational Circular Dichroism Spectroscopy. Dalton Trans. 2012, 41, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Morimoto, K.; Mori, Y.; Shinagawa, Y.; Kitazawa, T.; Yamagishi, A. Axially Chiral Monomeric and Dimeric Square Planar Pd(II) Complexes and their Application to Chiral Tectonics. Dalton Trans. 2013, 42, 7579–7585. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Taniguchi, T.; Monde, K.; Nishimura, S.-I.; Yamagishi, A. Dramatic Effects of d-Electron Configurations on Vibrational Circular Dichroism Spectra of Tris(acetylacetonato)metal(III). Chem. Lett. 2006, 35, 364–365. [Google Scholar] [CrossRef]
- Mori, H.; Yamagishi, A.; Sato, H. Theoretical Study on Vibrational Circular Dichroism Spectra of Tris(acetylacetonato)metal(III) Complexes: Anharmonic Effects and Low-lying Excited States. J. Chem. Phys. 2011, 135, 084506–084512. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, K.; Watanabe, Y.; Mori, S.; Sato, H. Vibrational Circular Dichroism and Single Crystal X-Ray Diffraction Analyses of [Ir(bzq)2(phen)]+ (bzq = benzo[h]quinoline; phen = 1,10-phenanthroline): Absolute Configuration and Role of CH-Interaction in Molecular Packing. Dalton Trans. 2017, 46, 4397–4402. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tamura, K.; Takimoto, K.; Yamagishi, A. Solid State Vibrational Circular Dichroism towards Molecular Recognition: Chiral Metal Complexes Intercalated in a Clay Mineral. Phys. Chem. Chem. Phys. 2018, 20, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Nafie, L.A. Infrared Vibrational Optical Activity: Measurement and Instrumentation. In Comprehensive Chiroptical Spectroscopy; Berova, N., Polavarapu, P.L., Nakanishi, K., Woody, R.W., Eds.; Wiley: Hoboken, NJ, USA, 2012; Volume 1, pp. 115–146. [Google Scholar]
- Stephen, P.J.; Lowe, M.A. Vibrational Circular Dichroism. Annu. Rev. Phys. Chem. 1985, 36, 213–241. [Google Scholar] [CrossRef]
- Wu, T.; Xiao-Zeng, Y.; Bour, P. Application of chirotical spectroscopy to coordination compounds. Coord. Chem. Rev. 2015, 284, 1–18. [Google Scholar] [CrossRef]
- Merten, C.; Hiller, K.; Xu, Y. Effects of Electron Configuration and Coordination Number on the Vibrational Circular Dichroism Spectra of Metal Complexes of Trans-1,2-diaminocyclohexane. Phys. Chem. Chem. Phys. 2012, 14, 12884–12891. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Terada, K.; Tsukube, H. Lanthanide Tris(β-diketonates) as Useful Probes for Chirality Determination of Biological Amino Alcohols in Vibrational Circular Dichroism: Ligand to Ligand Chirality Transfer in Lanthanide Coordination Spher. Chirality 2014, 26, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Domingos, S.R.; Huerta-Viga, A.; Baij, L.; Amirjalayer, S.; Dunnebier, D.A.E.; Walters, A.J.C.; Finger, M.; Nafie, L.A.; de Bruin, B.; Buma, W.J.; et al. Amplified Vibrational Circular Dichroism as a Probe of Local Biomolecular Structure. J. Am. Chem. Soc. 2014, 136, 3530–3535. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cao, X.; Nafie, L.A.; Freedman, T.B. Ab Initio VCD Calculation of a Transition-Metal Containing Molecule and a New Intensity Enhancement Mechanism for VCD. J. Am. Chem. Soc. 2001, 123, 11320–11321. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Yashima, E.; Hatada, K. Chromatographic resolution of tris(acetylacetonato)aluminium(III) on an optically active poly(triphenylmethyl methacrylate) column. J. Chem. Soc. Chem. Commun. 1984, 16, 1051–1052. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scurseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, A.; et al. Gaussian16; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]








| Temperature | CHCl3 (sec−1) | CH3OH (sec−1) |
|---|---|---|
| −10 | - | 2.05 × 10−5 |
| −5 | - | 3.31 × 10−5 |
| 0 | - | 8.78 × 10−5 |
| 5 | 3.77 × 10−6 | - |
| 10 | 8.36 × 10−6 | 3.04 × 10−4 |
| 15 | 2.28 × 10−5 | - |
| 20 | 4.91 × 10−5 | 1.03 × 10−3 |
| 25 | 1.06 × 10−4 | - |
| 30 | 2.39 × 10−4 | 2.65 × 10−3 |
| 35 | 6.32 × 10−4 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, H.; Sato, F.; Yamagishi, A. Chiral Tectonics: VCD and ECD Application for Epimerization of a Star-Burst Tetranuclear Complex with a Labile Central Core. Inorganics 2018, 6, 70. https://doi.org/10.3390/inorganics6030070
Sato H, Sato F, Yamagishi A. Chiral Tectonics: VCD and ECD Application for Epimerization of a Star-Burst Tetranuclear Complex with a Labile Central Core. Inorganics. 2018; 6(3):70. https://doi.org/10.3390/inorganics6030070
Chicago/Turabian StyleSato, Hisako, Fumi Sato, and Akihiko Yamagishi. 2018. "Chiral Tectonics: VCD and ECD Application for Epimerization of a Star-Burst Tetranuclear Complex with a Labile Central Core" Inorganics 6, no. 3: 70. https://doi.org/10.3390/inorganics6030070
APA StyleSato, H., Sato, F., & Yamagishi, A. (2018). Chiral Tectonics: VCD and ECD Application for Epimerization of a Star-Burst Tetranuclear Complex with a Labile Central Core. Inorganics, 6(3), 70. https://doi.org/10.3390/inorganics6030070