Chiral, Heterometallic Lanthanide–Transition Metal Complexes by Design
Abstract
:1. Introduction
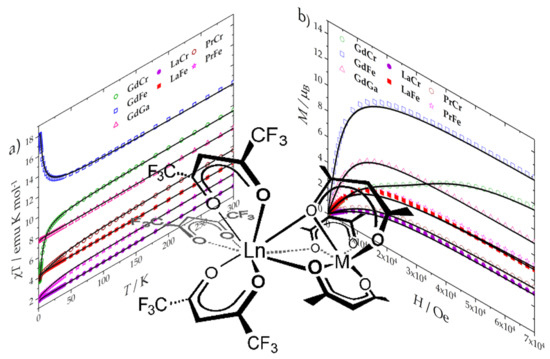
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis
3.3. Single Crystal X-ray Diffraction Measurements
3.4. Powder X-ray Diffraction Measurements
3.5. Magnetic Measurements
3.6. Simulations
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goodwin, C.A.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAdams, S.G.; Ariciu, A.-M.; Kostopoulos, A.K.; Walsh, J.P.; Tuna, F. Molecular single-ion magnets based on lanthanides and actinides: Design considerations and new advances in the context of quantum technologies. Coord. Chem. Rev. 2017, 346, 216–239. [Google Scholar] [CrossRef] [Green Version]
- Layfield, R.A.; Murugesu, M. Lanthanides and Actinides in Molecular Magnetism; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.; Layfield, R.A. Lanthanide single-molecule magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, F.; Cador, O.; Le Guennic, B.; Ouahab, L. Uncommon lanthanide ions in purely 4f single molecule magnets. Coord. Chem. Rev. 2017, 346, 150–175. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.L.; Wang, T.W.; Song, Y.; You, X.Z. Slow relaxation processes and single-ion magnetic behaviors in Dysprosium-containing complexes. Inorg. Chem. 2009, 49, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Chen, C.L.; Gao, Y.L.; Liu, X.L.; Feng, X.L.; Gui, Y.H.; Fang, S.M. Modulation of Homochiral DyIII Complexes: Single Molecule Magnets with Ferroelectric Properties. Chem. Eur. J. 2012, 18, 14632–14637. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Korobov, I.; Wernsdorfer, W.; Ungur, L.; Chibotaru, L.F.; Murugesu, M. A rare μ4-O Centred Dy4 Tetrahedron with Coordination Induced Local Chirality and Single Molecule Magnet Behaviour. Eur. J. Inorg. Chem. 2011, 10, 1535–1539. [Google Scholar] [CrossRef]
- Mihalcea, I.; Perfetti, M.; Pineider, F.; Tesi, L.; Mereacre, V.; Wilhelm, F.; Rogalev, A.; Anson, C.E.; Powell, A.K.; Sessoli, R. Spin Helicity in Chiral Lanthanide Chains. Inorg. Chem. 2016, 55, 10068–10074. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, B.M.; Bernhardt, P.V.; Krausz, E.R.; Lüthi, S.R.; Riley, M.J. A ligand-field analysis of the trensal (H3trensal = 2,2′,2″-tris(salicylideneimino)triethylamine) ligand. An application of the angular overlap model to lanthanides. Inorg. Chem. 2002, 41, 5024–5033. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Dreiser, J.; Weihe, H.; Sibille, R.; Johannesen, H.V.; Sørensen, M.A.; Nielsen, B.E.; Sigrist, M.; Mutka, H.; Rols, S. Design of single-molecule magnets: Insufficiency of the anisotropy barrier as the sole criterion. Inorg. Chem. 2015, 54, 7600–7606. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Ungur, L.; Sigrist, M.; Sundt, A.; Schau-Magnussen, M.; Vieru, V.; Mutka, H.; Rols, S.; Weihe, H.; Waldmann, O. Modifying the properties of 4f single-ion magnets by peripheral ligand functionalisation. Chem. Sci. 2014, 5, 1650–1660. [Google Scholar] [CrossRef] [Green Version]
- Perfetti, M.; Lucaccini, E.; Sorace, L.; Costes, J.P.; Sessoli, R. Determination of magnetic anisotropy in the lntrensal complexes (Ln = Tb, Dy, Er) by torque magnetometry. Inorg. Chem. 2015, 54, 3090–3092. [Google Scholar] [CrossRef] [PubMed]
- Lucaccini, E.; Baldoví, J.J.; Chelazzi, L.; Barra, A.-L.; Grepioni, F.; Costes, J.-P.; Sorace, L. Electronic structure and magnetic anisotropy in lanthanoid single-ion magnets with C3 symmetry: The Ln(trenovan) series. Inorg. Chem. 2017, 56, 4728–4738. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A. Magnetic polyoxometalates: From molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef] [PubMed]
- Baldoví, J.J.; Cardona-Serra, S.; Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A.; Palii, A. Rational design of single-ion magnets and spin qubits based on mononuclear lanthanoid complexes. Inorg. Chem. 2012, 51, 12565–12574. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Sugita, M.; Wernsdorfer, W. Quantum tunneling of magnetization in lanthanide single-molecule magnets: Bis(phthalocyaninato)terbium and bis(phthalocyaninato)dysprosium anions. Angew. Chem. Int. Ed. 2005, 44, 2931–2935. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.A.; Hansen, U.B.; Perfetti, M.; Pedersen, K.S.; Bartolomé, E.; Simeoni, G.G.; Mutka, H.; Rols, S.; Jeong, M.; Zivkovic, I.; et al. Chemical tunnel-splitting-engineering in a dysprosium-based molecular nanomagnet. Nat. Commun. 2018, 9, 1292. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.A.; Weihe, H.; Vinum, M.G.; Mortensen, J.S.; Doerrer, L.H.; Bendix, J. Imposing high-symmetry and tuneable geometry on lanthanide centres with chelating Pt and Pd metalloligands. Chem. Sci. 2017, 8, 3566–3575. [Google Scholar] [CrossRef] [Green Version]
- Benelli, C.; Caneschi, A.; Gatteschi, D.; Pardi, L.; Rey, P. Structure and magnetic properties of linear chain complexes of rare earth ions (Gadolinium, Europium) with nitronyl nitroxides. Inorg. Chem. 1989, 28, 275–280. [Google Scholar] [CrossRef]
- Gleizes, A.N.; Senocq, F.; Julve, M.; Sanz, J.L.; Kuzmina, N.; Troyanov, S.; Malkerova, I.; Alikhanyan, A.; Ryazanov, M.; Rogachev, A.; et al. Heterobimetallic single-source precursors for MOCVD. Synthesis and characterization of volatile mixed ligand complexes of Lanthanides, Barium and Magnesium beta-diketonates with d-element containing ligands. J. Phys. IV 1999, 9, 943–951. [Google Scholar]
- Evans, W.J.; Giarikos, D.G.; Johnston, M.A.; Greci, M.A.; Ziller, J.W. Reactivity of the europium hexafluoroacetylacetonate (hfac) complex, Eu(hfac)3(diglyme), and related analogs with potassium: Formation of the fluoride hfac “ate” complexes, [LnF(hfac)3K(diglyme)]2. J. Chem. Soc. Dalton Trans. 2002, 520–526. [Google Scholar] [CrossRef]
- Sun, O.; Gao, T.; Sun, J.; Li, G.; Li, H.; Xu, H.; Wang, C.; Yan, P. A series of lanthanide(III) complexes constructed from schiff base and β-diketonate ligands. CrystEngComm 2014, 16, 10460–10468. [Google Scholar] [CrossRef]
- Kennedy, F.; Shavaleev, N.M.; Koullourou, T.; Bell, Z.R.; Jeffery, J.C.; Faulkner, S.; Ward, M.D. Sensitised near-infrared luminescence from lanthanide(III) centres using Re(I) and Pt(II) diimine complexes as energy donors in d–f dinuclear complexes based on 2,3-bis(2-pyridyl)pyrazine. Dalton Trans. 2007, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Gleizes, A.; Julve, M.; Kuzmina, N.; Alikhanyan, A.; Lloret, F.; Malkerova, I.; Sanz, J.L.; Senocq, F. Heterobimetallic d–f metal complexes as potential single-source precursors for mocvd: Structure and thermodynamic study of the sublimation of [Ni(salen)Ln(hfa)3], Ln = Y, Gd. Eur. J. Inorg. Chem. 1998, 8, 1169–1174. [Google Scholar] [CrossRef]
- Pointillart, F.; Bernot, K.; Sessoli, R.; Gatteschi, D. Effects of 3d–4f magnetic exchange interactions on the dynamics of the magnetization of Dy(III)–M(II)–Dy(III) trinuclear clusters. Chem. Eur. J. 2007, 13, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Ramade, I.; Kahn, O.; Jeannin, Y.; Robert, F. Design and magnetic properties of a magnetically isolated GdIIICuII pair. Crystal structures of [Gd(hfa)3Cu(salen)], [Y(hfa)3Cu(salen)], [Gd(hfa)3Cu(salen)(meim)], and [La(hfa)3(H2O)Cu(salen)][hfa = Hexafluoroacetylacetonato, salen = N,N′-Ethylenebis (salicylideneaminato), meim = 1-Methylimidazole]. Inorg. Chem. 1997, 36, 930–936. [Google Scholar]
- Rogachev, A.Y.; Mironov, A.V.; Nemukhin, A.V. Experimental and theoretical studies of the products of reaction between Ln(hfa)3 band Cu (acac)2 (Ln = La, Y; acac = acetylacetonate, hfa = hexafluoroacetylacetonate). J. Mol. Struct. 2007, 831, 46–54. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sunatsuki, Y.; Ishida, H.; Kojima, M.; Akashi, H.; Re, N.; Matsumoto, N.; Pochaba, A.; Mrozinski, J. Synthesis, structures, and magnetic properties of face-sharing heterodinuclear Ni(II)–Ln(III) (Ln = Eu, Gd, Tb, Dy) complexes. Inorg. Chem. 2008, 47, 5736–5745. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Bernot, K. Rational organization of lanthanide-based smm dimers into three-dimensional networks. Inorg. Chem. 2015, 54, 5213–5219. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, S.-Y.; Shi, W.; Cheng, P.; Tang, J. Exploiting verdazyl radicals to assemble 2p–3d–4f one-dimensional chains. Dalton Trans. 2015, 44, 5364–5368. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Sun, J.; Li, L. Nitronyl nitroxide based 2p–3d–4f chains with the magnetocaloric effect and slow magnetic relaxation. Dalton Trans. 2015, 44, 18411–18417. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Hansen, S.; Lehmann, G. EPR of Fe3+ in acetylacetonates. Appl. Magn. Reson. 1990, 1, 47–53. [Google Scholar] [CrossRef]
- Pritchard, B.; Autschbach, J. Theoretical investigation of paramagnetic NMR shifts in transition metal acetylacetonato complexes: Analysis of signs, magnitudes, and the role of the covalency of ligand–metal bonding. Inorg. Chem. 2012, 51, 8340–8351. [Google Scholar] [CrossRef] [PubMed]
- Dreiser, J.; Pedersen, K.S.; Piamonteze, C.; Rusponi, S.; Salman, Z.; Ali, M.E.; Schau-Magnussen, M.; Thuesen, C.A.; Piligkos, S.; Weihe, H. Direct observation of a ferri-to-ferromagnetic transition in a fluoride-bridged 3d–4f molecular cluster. Chem. Sci. 2012, 3, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Cremades, E.; Gómez-Coca, S.; Aravena, D.; Alvarez, S.; Ruiz, E. Theoretical study of exchange coupling in 3d-Gd complexes: Large magnetocaloric effect systems. J. Am. Chem. Soc. 2012, 134, 10532–10542. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Sørensen, M.A.; Bendix, J. Fluoride-coordination chemistry in molecular and low-dimensional magnetism. Coord. Chem. Rev. 2015, 299, 1–21. [Google Scholar] [CrossRef]
- Holmberg, R.J.; Ungur, L.; Korobkov, I.; Chibotaru, L.; Murugesu, M. Observation of unusual slow-relaxation of the magnetisation in a Gd–EDTA chelate. Dalton Trans. 2015, 44, 20321–20325. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, T.C.; Wernsdorfer, W.; Christou, G. Enhancing the Quantum Properties of Manganese–Lanthanide Single-Molecule Magnets: Observation of Quantum Tunneling Steps in the Hysteresis Loops of a {Mn12Gd} Cluster. Angew. Chem. Int. Ed. 2009, 48, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Lucaccini, E.; Sorace, L.; Perfetti, M.; Costes, J.-P.; Sessoli, R. Beyond the anisotropy barrier: Slow relaxation of the magnetization in both easy-axis and easy-plane Ln(trensal) complexes. Chem. Commun. 2014, 50, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Bernot, K.; Pointillart, F.; Rosa, P.; Etienne, M.; Sessoli, R.; Gatteschi, D. Single molecule magnet behaviour in robust dysprosium–biradical complexes. Chem. Commun. 2010, 46, 6458–6460. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.F.; Wagner, W.F.; Sands, D.E. Rare-earth trishexafluoroacetylacetonates and related compounds. J. Inorg. Nucl. Chem. 1968, 30, 1275–1289. [Google Scholar] [CrossRef]
- Glidewell, C. Inorganic Experiments; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Liu, R.; Van Rooyen, P.H.; Conradie, J. Geometrical isomers of tris (β-diketonato) metal(III) complexes for M = Cr or Co: Synthesis, X-ray structures and DFT study. Inorg. Chim. Acta 2016, 447, 59–65. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. Easyspin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]






| Complex | M–Oacac/Å | Ln–Ohfac/Å | Ln–Oacac/Å | <O3>–<LnO3>–<MO3>/° a | Ln–M/Å |
|---|---|---|---|---|---|
| LaCr | 1.943–1.971 | 2.454–2.530 | 2.649–2.661 | 176.10 | 3.420 |
| LaFe | 1.962–2.049 | 2.457–2.503 | 2.629–2.636 | 177.21 | 3.514 |
| PrCr | 1.940–1.968 | 2.415–2.470 | 2.607–2.628 | 176.05 | 3.380 |
| PrFe | 1.958–2.045 | 2.426–2.467 | 2.587–2.606 | 177.38 | 3.471 |
| PrGa | 1.918–1.983 | 2.406–2.459 | 2.587–2.605 | 176.63 | 3.425 |
| GdCr | 1.936–1.966 | 2.336–2.399 | 2.535–2.565 | 176.18 | 3.321 |
| GdFe | 1.953–2.051 | 2.356–2.419 | 2.513–2.538 | 177.59 | 3.406 |
| GdGa | 1.921–1.983 | 2.336–2.406 | 2.516–2.530 | 176.80 | 3.355 |
| Sample | gLn | gM | DLn/cm−1 | DM/cm−1 | j/cm−1 |
|---|---|---|---|---|---|
| LaFe | - | 1.991 | - | −0.75 | - |
| LaCr | - | 1.965 | - | −0.98 | - |
| GdGa * | 1.980 | - | 0.0465 | - | - |
| GdFe | 1.980 | 1.991 | 0.0465 | −0.75 | −0.38 |
| GdCr | 1.980 | 1.965 | 0.0465 | −0.98 | +0.78 |
| PrGa | 4/5 | - | 80 | - | - |
| PrFe | 4/5 | 1.991 | 80 | −0.75 | −0.25 |
| PrCr | 4/5 | 1.965 | 80 | −0.98 | −1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Øwre, A.; Vinum, M.; Kern, M.; Van Slageren, J.; Bendix, J.; Perfetti, M. Chiral, Heterometallic Lanthanide–Transition Metal Complexes by Design. Inorganics 2018, 6, 72. https://doi.org/10.3390/inorganics6030072
Øwre A, Vinum M, Kern M, Van Slageren J, Bendix J, Perfetti M. Chiral, Heterometallic Lanthanide–Transition Metal Complexes by Design. Inorganics. 2018; 6(3):72. https://doi.org/10.3390/inorganics6030072
Chicago/Turabian StyleØwre, Anders, Morten Vinum, Michal Kern, Joris Van Slageren, Jesper Bendix, and Mauro Perfetti. 2018. "Chiral, Heterometallic Lanthanide–Transition Metal Complexes by Design" Inorganics 6, no. 3: 72. https://doi.org/10.3390/inorganics6030072
APA StyleØwre, A., Vinum, M., Kern, M., Van Slageren, J., Bendix, J., & Perfetti, M. (2018). Chiral, Heterometallic Lanthanide–Transition Metal Complexes by Design. Inorganics, 6(3), 72. https://doi.org/10.3390/inorganics6030072