Synthesis and Characterization of the Germathioacid Chloride Coordinated by an N-Heterocyclic Carbene §
Abstract
:1. Introduction
2. Results and Discussion
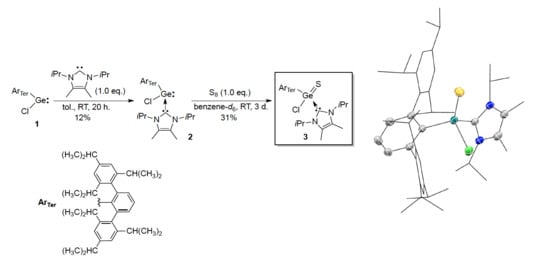
2.1. Synthesis and Structure of Germathioacid Chloride 3
2.2. Density Functional Theory Studies on the Germathioacid Chloride 3
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of Ge(Cl){C6H3-2,6-Tip2}(Im-i-Pr2Me2) 2
3.2.2. Synthesis of Ge(S)Cl{C6H3-2,6-Tip2}(Im-i-Pr2Me2) 3
3.3. Single-Crystal X-ray Analysis of Ge(S)Cl{C6H3-2,6-Tip2}(Im-i-Pr2Me2) 3
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Power, P.P. Main-group elements as transition metal. Nature 2010, 463, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.Y.; Sekiguchi, A. Organometallic Compounds of Low Coordinate Si, Ge, Sn and Pb: From Phantom Species to Stable Compounds; Wiley: Chichester, UK, 2010. [Google Scholar]
- Tokitoh, N.; Okazaki, R. Recent advances in the chemistry of group 14–group 16 double bond compound. Adv. Organomet. Chem. 2001, 47, 121–166. [Google Scholar]
- Brook, A.G.; Abdesaken, F.; Gutekunst, B.; Gutekunst, G.; Kallury, R.K. A solid silaethene: Isolation and characterization. J. Chem. Soc. Chem. Commun. 1981, 4, 191–192. [Google Scholar] [CrossRef]
- West, R.; Fink, M.J.; Michl, J. Tetramesityldisilene, a stable compoundd containing a silicon-silicon double bond. Science 1981, 214, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Yoshifuji, M.; Shima, I.; Inamoto, N.; Hirotsu, K.; Higuchi, T. Synthesis and structure of bis(2,4,6-tri-tert-buthylphenyl)diphosphene: Isolation of a true phosphobenzene. J. Am. Chem. Soc. 1981, 103, 4587–4589. [Google Scholar] [CrossRef]
- Okazaki, R.; Tokitoh, N. Heavy Ketones, the Heavier Element Congeners of a Ketone. Acc. Chem. Res. 2000, 33, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.C.; Power, P.P. π-Bonding and the Lone Pair Effect in Multiple Bonds involving Heavier Main Group Elements: Developments in the New Millennium. Chem. Rev. 2010, 110, 3877–3923. [Google Scholar] [CrossRef] [PubMed]
- Veith, M.; Becker, S.; Huch, V. A Base-Stabilized Ge–S Double Bond. Angew. Chem. Int. Ed. Engl. 1989, 28, 1237–1238. [Google Scholar] [CrossRef]
- Tokitoh, N.; Matsumoto, T.; Manmaru, K.; Okazaki, R. Synthesis and Crystal Structure of the First Stable Diarylgermanetione. J. Am. Chem. Soc. 1993, 115, 8855–8856. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tokitoh, N.; Okazaki, R. The First Kinetically Stabilized Germanethiones and Germaneselones: Syntheses, Structure, and Reactivities. J. Am. Chem. Soc. 1999, 121, 8811–8824. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, Q.; Usón, I.; Roesky, H.W.; Noltemeyer, M.; Schmidt, H.-G. Synthesis and Structures of [{HC(CMeNAr)2}Ge(S)X] (Ar = 2,6-iPr2C6H3, X = F, Cl, Me): Structurally Characterized Examples with a Formal Double Bond between Group 14 and 16 Elements Bearing a Halide. J. Am. Chem. Soc. 2002, 124, 8542–8543. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, B.; Singh, S. Concise access to iminophosphonamide stabilized heteroleptic germylenes: Chemical reactivity and structural investigation. Dalton Trans. 2016, 45, 6079–6087. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.-P.; Chong, K.-H.; Wu, Y.-S.; So, C.-W.; Chan, H.-S.; Mak, T.C.W. Synthesis of Chalcogeno[3-(pyrid-2-yl)-1-azaallyl]germanium Complexes. Eur. J. Inorg. Chem. 2006, 808–812. [Google Scholar] [CrossRef]
- Sinhababu, S.; Siwatch, R.K.; Mukherjee, G.; Rajaraman, G.; Nagendran, S. Aminotroponiminatogermaacid Halides with a Ge(E)X Moiety (E = S, Se; X = F, Cl). Inorg. Chem. 2012, 51, 9240–9248. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yao, S.; Inoue, S.; Berkefeld, A.; Driess, M. Taming the germyliumylidene [ClGe:]+ and germathionium [ClGe=S]+ ions by donor–acceptor stabilization using 1,8-bis(tributylphosphazenyl)naphthalene. Chem. Commun. 2012, 48, 12198–12200. [Google Scholar] [CrossRef] [PubMed]
- Filippou, A.C.; Chernov, O.; Blom, B.; Stumpf, K.W.; Schnakenburg, G. Stable N-Heterocyclic Carbene Adducts of Arylchlorosilylenes and Their Germanium Homologues. Chem. Eur. J. 2010, 16, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Ossig, G.; Meller, A.; Brönneke, C.; Müller, O.; Schäfer, M.; Herbst-Irmer, R. Bis[(2-pyridyl)bis(trimethylsilyl)methyl-C,N]germanium(II): A Base-Stabilized Germylene and the Corresponding Germanethione, Germaneselenone, and Germanetellurone. Organometallics 1997, 16, 2116–2120. [Google Scholar] [CrossRef]
- Li, L.; Fukawa, T.; Matsuo, T.; Hashizume, D.; Fueno, H.; Tanaka, K.; Tamao, K. A stable germanone as the first isolated heavy ketone with a terminal oxygen atom. Nat. Chem. 2012, 4, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Schiemenz, B.; Power, P.P. Synthesis of Sterically Encumbered Terphenyls and Characterization of Their Metal Derivatives Et2OLiC6H3-2,6-Trip2 and Me2SCuC6H3-2,6-Trip2 (Trip = 2,4,6-i-Pr3C6H2-). Organometallics 1996, 15, 958–964. [Google Scholar] [CrossRef]
- Pu, L.; Olmstead, M.M.; Power, P.P. Synthesis and Characterization of the Monomeric Terphenyl-Metal Halides Ge(Cl){C6H3-2,6-Trip2}(Trip = C6H2-2,4,6-i-Pr3) and Sn(I){C6H3-2,6-Trip2} and the Terphenyl-Metal Amide Sn{N(SiMe3)2}{C6H3-2,6-Trip2}. Organometallics 1998, 17, 5602–5606. [Google Scholar] [CrossRef]
- Kuhn, N.; Kratz, Y. Synthesis of Imidazol-2-ylidenes by Reduction of Imidazole-2-(3H)-thiones. Synthesis 1993, 561–562. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97; Program for the Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Kabuto, C.; Akine, S.; Nemoto, T.; Kwon, E. Release of Software (Yadokari-XG 2009) for Crystal Structure Analyses. J. Cryst. Soc. Jpn. 2009, 51, 218–224. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Sizova, O.V.; Skripnikov, L.V.; Sokolv, A.Y. Symmetry decomposition of quantum chemical bond orders. THEOCHEM 2008, 870, 1–9. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egawa, Y.; Fukumoto, C.; Mikami, K.; Takeda, N.; Unno, M. Synthesis and Characterization of the Germathioacid Chloride Coordinated by an N-Heterocyclic Carbene §. Inorganics 2018, 6, 76. https://doi.org/10.3390/inorganics6030076
Egawa Y, Fukumoto C, Mikami K, Takeda N, Unno M. Synthesis and Characterization of the Germathioacid Chloride Coordinated by an N-Heterocyclic Carbene §. Inorganics. 2018; 6(3):76. https://doi.org/10.3390/inorganics6030076
Chicago/Turabian StyleEgawa, Yasunobu, Chihiro Fukumoto, Koichiro Mikami, Nobuhiro Takeda, and Masafumi Unno. 2018. "Synthesis and Characterization of the Germathioacid Chloride Coordinated by an N-Heterocyclic Carbene §" Inorganics 6, no. 3: 76. https://doi.org/10.3390/inorganics6030076
APA StyleEgawa, Y., Fukumoto, C., Mikami, K., Takeda, N., & Unno, M. (2018). Synthesis and Characterization of the Germathioacid Chloride Coordinated by an N-Heterocyclic Carbene §. Inorganics, 6(3), 76. https://doi.org/10.3390/inorganics6030076