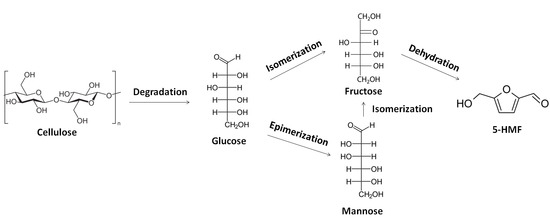
Metal-Catalyzed Degradation of Cellulose in Ionic Liquid Media
Abstract
:1. Introduction
- strong Brønsted acidity;
- the capability to activate oxidants such as O2 and H2O2 for selective oxidation;
- high water tolerance;
- tunable acidity, redox potential, and solubility in various media, which allow the rational design of active sites on molecular and atomic scales;
- high thermal and oxidative stability as compared with common molecular catalysts such as organometallic complexes and enzymes;
- ease of handling and separation, and the relatively low corrosiveness, possibly owing to the generated corrosion-inhibiting films, which allow them to act as environmentally friendly liquid-phase catalysts, unlike mineral acids.
2. Results and Discussion
2.1. Reaction Time for POM-IL Catalysts
2.2. Cellulose Loading and the Efficiency of POM Formation
2.3. Effect of the Water Content of the Reaction Medium on the Conversion of Cellulose to HMF
3. Materials and Methods
3.1. Catalytic Formation of HMF from Cellulose
3.2. Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nanda, S.; Azargohar, R.; Dalai, A.K.; Kozinski, J.A. An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew. Sustain. Energy Rev. 2015, 50, 925–941. [Google Scholar] [CrossRef]
- Sanderson, K. Lignocellulose: A chewy problem. Nature 2011, 474, S12–S14. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Matute, A.I.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Derivatization of carbohydrates for GC and GC-MS analyses. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, S.; Huang, J.; Chen, L.; Ma, L.; Huang, X. Conversion of cellulose and cellobiose into sorbitol catalyzed by ruthenium supported on a polyoxometalate/metal-organic framework hybrid. ChemSusChem 2013, 6, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- To, C.R. Cutting-edge research for a greener sustainable future. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Eminov, S.; Filippousi, P.; Brandt, A.; Wilton-Ely, J.; Hallett, J. Direct Catalytic Conversion of Cellulose to 5-Hydroxymethylfurfural Using Ionic Liquids. Inorganics 2016, 4, 32. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-łukasik, E.; Bogel-łukasik, R. Ionic Liquid-Mediated Formation of 5-Hydroxymethylfurfural—A Promising Biomass-Derived Building Block. Chem. Rev. 2010, 111, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, S.; Mikkola, J.P.; Murzin, D.Y.; Vaher, M.; Kaljurand, M.; Koel, M. Sugars and sugar derivatives in ionic liquid media obtained from lignocellulosic biomass: Comparison of capillary electrophoresis and chromatographic analysis. Catal. Today 2014, 223, 18–24. [Google Scholar] [CrossRef]
- Rinaldi, R.; Palkovits, R.; Schüth, F. Depolymerization of Cellulose Using Solid Catalysts in Ionic Liquids. Angew. Chem. 2008, 120, 8047–8050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, H.; Qian, X.; Chen, E.Y. Ionic Liquid–Water Mixtures: Enhanced Kw for Efficient Cellulosic Biomass Conversion. Energy Fuels 2010, 24, 2410–2417. [Google Scholar] [CrossRef]
- Parveen, F.; Patra, T.; Upadhyayula, S. A structure—Activity relationship study using DFT analysis of Bronsted—Lewis acidic ionic liquids and synergistic effect of dual acidity in one-pot. New J. Chem. 2018, 42, 1423–1430. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Hossain, S.A.; Islam, T.; Alamri, H.R. Direct Production of Furfural in One-pot Fashion from Raw Biomass Using Brønsted Acidic Ionic Liquids. Sci. Rep. 2017, 7, 13508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Jing, Y.; Liu, C.; Zhang, D. A theoretical elucidation: Why does a SO3H-functionalized imidazolium-based ionic liquid catalyze the conversion of 5-hydroxymethylfurfural to levulinic acid? New J. Chem. 2017, 41, 8714–8720. [Google Scholar] [CrossRef]
- Du, X.; Zhang, J.; Wang, Y.; Qu, Y. Conversion of Carbohydrates into Platform Chemicals Catalyzed by Alkaline Ionic Liquids. Catalysts 2017, 7, 245. [Google Scholar] [CrossRef]
- Omwoma, S.; Gore, C.T.; Ji, Y.; Hu, C.; Song, Y. Environmentally benign polyoxometalate materials. Coord. Chem. Rev. 2015, 286, 17–29. [Google Scholar] [CrossRef]
- Weiping, W.Y.D.; Qinghong, Z. Polyoxometalates as efficient catalysts for transformations of cellulose into platform chemicals. Dalt. Trans. 2012, 41, 9817–9831. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.; Chen, L. Efficient production of 5-hydroxymethylfurfural and alkyl levulinate from biomass carbohydrate using ionic liquid-based polyoxometalate salts. RSC Adv. 2014, 4, 4194–4202. [Google Scholar] [CrossRef]
- Abia, J.A.; Ozer, R. Development of polyoxometalate-ionic liquid compounds for processing cellulosic biomass. BioResources 2013, 8, 2924–2933. [Google Scholar] [CrossRef]
- Chidambaram, M.; Bell, A.T. A two-step approach for the catalytic conversion of glucose to 2,5-dimethylfuran in ionic liquids. Green Chem. 2010, 12, 1253–1262. [Google Scholar] [CrossRef]
- Aid, T.; Paist, L.; Lopp, M.; Kaljurand, M.; Vaher, M. An optimized capillary electrophoresis method for the simultaneous analysis of biomass degradation products in ionic liquid containing samples. J. Chromatogr. A 2016, 1447, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Aid, T.; Hyvärinen, S.; Vaher, M.; Koel, M.; Mikkola, J.P. Saccharification of lignocellulosic biomasses via ionic liquid pretreatment. Ind. Crop. Prod. 2016, 92, 336–341. [Google Scholar] [CrossRef]
- Megías-sayago, C.; Álvarez, E. Epimerization of glucose over ionic liquid/Phosphomolybdate hybrids: Structure—Activity relationship. Green Chem. 2018, 20, 1042–1049. [Google Scholar] [CrossRef]
- Bermejo-Deval, R.; Orazov, M.; Gounder, R.; Hwang, S.J.; Davis, M.E. Active sites in Sn-beta for glucose isomerization to fructose and epimerization to mannose. ACS Catal. 2014, 4, 2288–2297. [Google Scholar] [CrossRef]
- Bermejo-Deval, R.; Assary, R.S.; Nikolla, E.; Moliner, M.; Roman-Leshkov, Y.; Hwang, S.J.; Palsdottir, A.; Silverman, D.; Lobo, R.F.; Curtiss, L.A.; et al. Metalloenzyme-like catalyzed isomerizations of sugars by Lewis acid zeolites. Proc. Natl. Acad. Sci. USA 2012, 109, 9727–9732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gounder, R.; Davis, M.E. Monosaccharide and disaccharide isomerization over Lewis acid sites in hydrophobic and hydrophilic molecular sieves. J. Catal. 2013, 308, 176–188. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, R.; Ma, Z.; Wu, T.; Wu, Y. Conversion of cellulose to HMF in ionic liquid catalyzed by bifunctional ionic liquids. Bioresour. Technol. 2013, 129, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Tofanelli, M.A.; Ernst, R.D.; Eyring, E.M. Chromium (III) catalysts in ionic liquids for the conversion of glucose to 5-(hydroxymethyl)furfural (HMF): Insight into metal catalyst:ionic liquid mediated conversion of cellulosic biomass to biofuels and chemicals. Biomass Bioenergy 2012, 42, 224–227. [Google Scholar] [CrossRef]
- Tadesse, H.; Luque, R. Advances on biomass pretreatment using ionic liquids: An overview. Energy Environ. Sci. 2011, 4, 3913. [Google Scholar] [CrossRef]
- Li, G.; Pidko, E.A.; Hensen, E.J.M. A Periodic DFT Study of Glucose to Fructose Isomerization on Tungstite (WO3 H2O): Influence of Group IV–VI Dopants and Cooperativity with Hydroxyl Groups. ACS Catal. 2016, 6, 4162–4169. [Google Scholar] [CrossRef]
- Ju, F.; Vandervelde, D.; Nikolla, E. Molybdenum-Based Polyoxometalates as Highly Active and Selective Catalysts for the Epimerization of Aldoses. ACS Catal. 2014, 4, 1358–1364. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; Nikolakis, V.; Vlachos, D.G. Mechanistic Insights into Lewis Acid Metal Salt-Catalyzed Glucose Chemistry in Aqueous Solution. ACS Catal. 2016, 6, 1497–1504. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, J.; Elgasim, A.; Yagoub, A.; Ma, H. Conversion of glucose into 5-hydroxymethylfurfural in different solvents and catalysts: Reaction kinetics and mechanism. Egypt. J. Pet. 2017, 26, 477–487. [Google Scholar] [CrossRef]
- Weingarten, R.; Tae, Y.; Tompsett, G.A.; Fernández, A.; Sung, K.; Hagaman, E.W.; Curt, W.; Dumesic, J.A.C., Jr.; Huber, G.W. Conversion of glucose into levulinic acid with solid metal (IV) phosphate catalysts. J. Catal. 2013, 304, 123–134. [Google Scholar] [CrossRef]
- Torres, A.I.; Daoutidis, P.; Tsapatsis, M. Continuous production of 5-hydroxymethylfurfural from fructose: A design case study. Energy Environ. Sci. 2010, 3, 1560. [Google Scholar] [CrossRef]
- Ivanova, S. Hybrid organic–inorganic materials based on polyoxometalates. ISRN Chem. Eng. 2014, 2014, 963792. [Google Scholar] [CrossRef]






| Catalyst | Glucose, % | Mannose, % | HMF, % | Total Products Yield, % |
|---|---|---|---|---|
| No catalyst | >0.04 | >0.04 | >0.04 | >0.04 |
| ZnCl2 | >0.04 | >0.04 | 4.64 | 4.64 |
| MgCl2 | >0.04 | >0.04 | 3.78 | 3.78 |
| CrCl3 | >0.04 | >0.04 | 55.3 | 55.3 |
| W-POM | 33.7 | >0.04 | 5.82 | 39.5 |
| Mo-POM | 4.96 | 2.26 | 2.95 | 10.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aid, T.; Koel, M.; Lopp, M.; Vaher, M. Metal-Catalyzed Degradation of Cellulose in Ionic Liquid Media. Inorganics 2018, 6, 78. https://doi.org/10.3390/inorganics6030078
Aid T, Koel M, Lopp M, Vaher M. Metal-Catalyzed Degradation of Cellulose in Ionic Liquid Media. Inorganics. 2018; 6(3):78. https://doi.org/10.3390/inorganics6030078
Chicago/Turabian StyleAid, Tiina, Mihkel Koel, Margus Lopp, and Merike Vaher. 2018. "Metal-Catalyzed Degradation of Cellulose in Ionic Liquid Media" Inorganics 6, no. 3: 78. https://doi.org/10.3390/inorganics6030078
APA StyleAid, T., Koel, M., Lopp, M., & Vaher, M. (2018). Metal-Catalyzed Degradation of Cellulose in Ionic Liquid Media. Inorganics, 6(3), 78. https://doi.org/10.3390/inorganics6030078