High-Throughput Synthesis of Pillared-Layered Magnesium Tetraphosphonate Coordination Polymers: Framework Interconversions and Proton Conductivity Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Behavior
2.2. Crystal Structures
2.3. Gas Adsorption
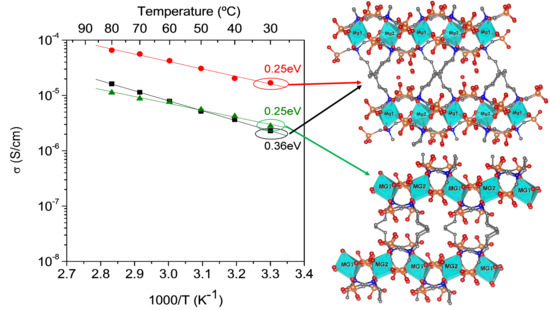
2.4. Proton Conductivity
3. Materials and Methods
3.1. General Information
3.2. Small Scale-Synthesis: High-Throughput Study
3.3. Synthesis Scale-Up
3.4. Microwave-Assisted Synthesis
3.5. Gas Adsorption Characterization
3.6. Structural Characterization
3.7. Conductivity Characterization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clearfield, A.; Demadis, K.D. Metal Phosphonate Chemistry: From Synthesis to Applications; Royal Society of Chemistry: Cambridge, UK, 2012; ISBN -13. [Google Scholar]
- Moedritzer, K.; Irani, R.R. The Direct Synthesis of α-Aminomethylphosphonic Acids Mannich-Type Reactions with Orthophosphorous. Acid. J. Org. Chem. 1966, 31, 1603–1607. [Google Scholar] [CrossRef]
- Cardiano, P.; Cigala, R.M.; Cordaro, M.; De Stefano, C.; Milea, D.; Sammartano, S. On the complexation of metal cations with “pure” diethylenetriamine-N,N,N′,N″,N″-pentakis(methylenephosphonic) acid. New J. Chem. 2017, 41, 4065–4075. [Google Scholar] [CrossRef]
- Villemin, D.; Didi, M.A. Aminomethylenephosphonic Acids Syntheses and Applications (A Review). Orient. J. Chem. 2015, 31, 1–12. [Google Scholar] [CrossRef]
- Chirby, D.; Franck, S.; Troutner, D.E. Adsorption of 153Sm-EDTMP on calcium hydroxyapatite. Int. J. Radiat. Appl. Instrum. Part A 1988, 39, 495–499. [Google Scholar] [CrossRef]
- Demadis, K.D.; Barouda, E.; Stavgianoudaki, N.; Zhao, H. Inorganic-Organic Hybrid Molecular “Ribbons” Based on Chelating/Bridging, “Pincer” Tetraphosphonates and Alkaline-Earth Metals. Cryst. Growth Des. 2009, 9, 1250–1253. [Google Scholar] [CrossRef]
- Demadis, K.D.; Mantzaridis, C.; Lykoudis, P. Effects of Structural Differences on Metallic Corrosion Inhibition by Metal−Polyphosphonate Thin Films. Ind. Eng. Chem. Res. 2006, 45, 7795–7800. [Google Scholar] [CrossRef]
- Taddei, M.; Costantino, F.; Ienco, A.; Comotti, A.; Dau, P.V.; Cohen, S.M. Synthesis, breathing, and gas sorption study of the first isoreticular mixed-linker phosphonate based metal–organic frameworks. Chem. Commun. 2013, 49, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Colodrero, R.M.P.; Cabeza, A.; Olivera-Pastor, P.; Infantes-Molina, A.; Barouda, E.; Demadis, K.D.; Aranda, M.A.G. “Breathing” in Adsorbate-Responsive Metal Tetraphosphonate Hybrid Materials. Chem. Eur. J. 2009, 15, 6612–6618. [Google Scholar] [CrossRef] [PubMed]
- Colodrero, R.M.P.; Olivera-Pastor, P.; Losilla, E.R.; Aranda, M.A.G.; Papadaki, M.; McKinlay, A.; Morris, R.E.; Demadis, K.D.; Cabeza, A. Multifunctional Lanthanum Tetraphosphonates: Flexible, Ultramicroporous and Proton-Conducting Hybrid Frameworks. Dalton Trans. 2012, 41, 4045–4051. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.F.; Ananias, D.; Carlos, L.D.; Rocha, J.; Almeida Paz, F.A. Photoluminescent Lanthanide-Organic Framework Based on a Tetraphosphonic Acid Linker. Cryst. Growth Des. 2017, 17, 5191–5199. [Google Scholar] [CrossRef]
- Colodrero, R.M.P.; Olivera-Pastor, P.; Losilla, E.R.; Hernandez-Alonso, D.; Aranda, M.A.G.; Leon-Reina, L.; Rius, J.; Demadis, K.D.; Moreau, B.; Villemin, D.; et al. High Proton Conductivity in a Flexible, Cross-Linked, Ultramicroporous Magnesium Tetraphosphonate Hybrid Framework. Inorg. Chem. 2012, 51, 7689–7698. [Google Scholar] [CrossRef] [PubMed]
- Schütrumpf, A.; Duthie, A.; Lork, E.; Yücesan, G.; Beckmann, J. Synthesis of Some Di- and Tetraphosphonic Acids by Suzuki Cross-Coupling. Z. Anorg. Allg. Chem. 2018, in press. [Google Scholar] [CrossRef]
- Feyand, M.; Seidler, C.F.; Deiter, C.; Rothkirch, A.; Lieb, A.; Wark, M.; Stock, N. High-throughput Microwave-assisted Discovery of New Metal Phosphonates. Dalton Trans. 2013, 42, 8761–8770. [Google Scholar] [CrossRef] [PubMed]
- Colodrero, R.M.P.; Angeli, G.K.; Bazaga-Garcia, M.; Olivera-Pastor, P.; Villemin, D.; Losilla, E.R.; Martos, E.Q.; Hix, G.B.; Aranda, M.A.G.; Demadis, K.D.; et al. Structural Variability in Multifunctional Metal Xylenediaminetetraphosphonate Hybrids. Inorg. Chem. 2013, 52, 8770–8783. [Google Scholar] [CrossRef] [PubMed]
- Firmino, A.D.G.; Mendes, R.F.; Antunes, M.M.; Barbosa, P.C.; Vilela, S.M.F.; Valente, A.A.; Figueiredo, F.M.L.; Tomé, J.P.C.; Almeida Paz, F.A. Robust Multifunctional Yttrium-Based Metal−Organic Frameworks with Breathing Effect. Inorg. Chem. 2017, 56, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Schütrumpf, A.; Bulut, A.; Hermer, N.; Zorlu, Y.; Kirpi, E.; Stock, N.; Yazaydın, A.O.; Yücesan, G.; Beckmann, J. From Tetrahedral Tetraphosphonic Acids E[p-C6H4P(O)(OH)2]4 (E = C, Si) to Porous Cu–and Zn–MOFs with Large Surface Areas. ChemistrySelect 2017, 2, 3035–3038. [Google Scholar] [CrossRef]
- Zaręba, J.K.; Białek, M.J.; Janczak, J.; Nyk, M.; Zoń, J.; Samoć, M. Beyond Single-Wavelength SHG Measurements: Spectrally-Resolved SHG Studies of Tetraphosphonate Ester Coordination Polymers. Inorg. Chem. 2015, 54, 10568–10575. [Google Scholar] [CrossRef] [PubMed]
- Zaręba, J.K. Tetraphenylmethane and tetraphenylsilane as building units of coordination polymers and supramolecular networks—A focus on tetraphosphonates. Inorg. Chem. Commun. 2017, 86, 172–186. [Google Scholar] [CrossRef]
- Rhauderwiek, T.; Wolkersdörfer, K.; Øien-Ødegaard, S.; Lillerud, K.-P.; Wark, M.; Stock, N. Crystalline and permanently porous porphyrin-based metal tetraphosphonates. Chem. Commun. 2018, 54, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Firmino, A.D.G.; Figueira, F.; Tomé, J.P.C.; Almeida Paz, F.A.; Rocha, J. Metal–Organic Frameworks assembled from tetraphosphonic ligands and lanthanides. Coord. Chem. Rev. 2018, 355, 133–149. [Google Scholar] [CrossRef]
- Plabst, M.; McCusker, L.B.; Bein, T. Exceptional Ion-Exchange Selectivity in a Flexible Open Framework Lanthanum(III)tetrakisphosphonate. J. Am. Chem. Soc. 2009, 131, 18112–18118. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Rauscher, M.; Bein, T. Inorganic–organic hybrid compounds: Hydrothermal synthesis and characterization of a new three-dimensional metal tetraphosphonate Mn[(HO3PCH2)N(H)(CH2)4(H)N(CH2PO3H)2]. J. Solid State Chem. 2004, 177, 642–647. [Google Scholar] [CrossRef]
- Demadis, K.D.; Mantzaridis, C.; Raptis, R.G.; Mezei, G. Metal–Organotetraphosphonate Inorganic–Organic Hybrids: Crystal Structure and Anticorrosion Effects of Zinc Hexamethylenediaminetetrakis(methylenephosphonate) on Carbon Steels. Inorg. Chem. 2005, 44, 4469–4471. [Google Scholar] [CrossRef] [PubMed]
- Demadis, K.D.; Barouda, E.; Zhao, H.; Raptis, R.G. Structural architectures of charge-assisted, hydrogen-bonded, 2D layered aminetetraphosphonate and zinctetraphosphonate ionic materials. Polyhedron 2009, 28, 3361–3367. [Google Scholar] [CrossRef]
- Mondry, A.; Janicki, R. From structural properties of the EuIII complex with ethylenediaminetetra(methylenephosphonic acid) (H8EDTMP) towards biomedical applications. Dalton Trans. 2006, 0, 470–4710. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hou, H.; Han, H.; Fan, Y. Highly Selective Ferric Ion Sorption and Exchange by Crystalline Metal Phosphonates Constructed from Tetraphosphonic Acids. Inorg. Chem. 2007, 46, 7960–7970. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Colomban, P.; Novak, A. Proton Transfer and Superionic Conductivity in Solids and Gels. J. Mol. Struct. 1988, 177, 277–308. [Google Scholar] [CrossRef]
- Villemin, D.; Moreau, B.; Elbilali, A.; Didi, M.-A.; Kaid, M.; Jaffrès, P.-A. Green Synthesis of Poly(aminomethylenephosphonic) Acids. Phosphorus Sulfur 2010, 185, 2511–2519. [Google Scholar] [CrossRef]
- Colodrero, R.M.P.; Olivera-Pastor, P.; Cabeza, A.; Papadaki, M.; Demadis, K.D.; Aranda, M.A.G. Structural Mapping and Framework Interconversions in 1D, 2D, and 3D Divalent Metal R,S-Hydroxyphosphonoacetate Hybrids. Inorg. Chem. 2010, 49, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Colodrero, R.M.P.; Cabeza, A.; Olivera-Pastor, P.; Rius, J.; Choquesillo-Lazarte, D.; García-Ruiz, J.M.; Papadaki, M.; Demadis, K.D.; Aranda, M.A.G. Common Structural Features in Calcium Hydroxyphosphonoacetates. A High-Throughput Screening. Cryst. Growth Des. 2011, 11, 1713–1722. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. USSR 1947, 55, 331–333. [Google Scholar]
- Boultif, A.; Louer, D. Powder pattern indexing with the dichotomy method. J. Appl. Cryst. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Vallcorba, O.; Rius, J.; Frontera, C.; Peral, I.; Miravitlles, C. DAJUST: A suite of computer programs for pattern matching, space-group determination and intensity extraction from powder diffraction data. J. Appl. Cryst. 2012, 45, 844–848. [Google Scholar] [CrossRef]
- Rius, J. Patterson-function direct methods for structure determination of organic compounds from powder diffraction data. XVI. Acta Cryst. 2011, A67, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M.A. profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR 86–748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2004. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- WinDETA; v 5.66; Novocontrol GmbH: Hundsangen, Germany, 1995.








| Compound | 1 | 1deh | 2 |
|---|---|---|---|
| Chemical formula | MgP4O13N2C10H28 | MgP4O12N2C10H26 | MgP4O12N2C10H26 |
| Formula Mass | 532.54 | 514.52 | 514.52 |
| Crystal system | Triclinic | Monoclinic | Triclinic |
| Space Group | P | C2/c | P |
| λ/Å | 1.5406 | 1.5406 | 1.5406 |
| a/Å | 12.5276(3) | 23.469(1) | 12.362(2) |
| b/Å | 9.70223(2) | 8.6872(4) | 9.0720(1) |
| c/Å | 8.62223(2) | 9.6165(5) | 9.552501) |
| α/° | 91.917(1) | 90.0 | 90.6651(8) |
| β/° | 70.621(1) | 99.219(4) | 106.7290(9) |
| γ/° | 86.485(2) | 90.0 | 111.8975(8) |
| Unit cell volume/Å3 | 985.16(4) | 1935.3(2) | 943.45(2) |
| Z | 2 | 4 | 2 |
| Vnon-H-atom/Å3 | 16.42 | 16.68 | 16.27 |
| Temperature/K | 298 | 493 | 298 |
| No. independent reflections | 2043 | 588 | 1947 |
| Data/Restrains/Parameters | 5710/65/132 | 4147/33/75 | 5398/63/135 |
| RWP | 0.0474 | 0.0812 | 0.0617 |
| RP | 0.0361 | 0.0602 | 0.0482 |
| RF | 0.0654 | 0.0500 | 0.0425 |
| CCDC number | 1855415 | 1855416 | 1855417 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colodrero, R.M.P.; Salcedo, I.R.; Bazaga-García, M.; Barouda, E.; Papadaki, M.; Papathanasiou, K.E.; Hernández-Alonso, D.; Rius, J.; Aranda, M.A.G.; Losilla, E.R.; et al. High-Throughput Synthesis of Pillared-Layered Magnesium Tetraphosphonate Coordination Polymers: Framework Interconversions and Proton Conductivity Studies. Inorganics 2018, 6, 96. https://doi.org/10.3390/inorganics6030096
Colodrero RMP, Salcedo IR, Bazaga-García M, Barouda E, Papadaki M, Papathanasiou KE, Hernández-Alonso D, Rius J, Aranda MAG, Losilla ER, et al. High-Throughput Synthesis of Pillared-Layered Magnesium Tetraphosphonate Coordination Polymers: Framework Interconversions and Proton Conductivity Studies. Inorganics. 2018; 6(3):96. https://doi.org/10.3390/inorganics6030096
Chicago/Turabian StyleColodrero, Rosario M.P., Inés R. Salcedo, Montse Bazaga-García, Eleni Barouda, Maria Papadaki, Konstantinos E. Papathanasiou, Daniel Hernández-Alonso, Jordi Rius, Miguel A.G. Aranda, Enrique R. Losilla, and et al. 2018. "High-Throughput Synthesis of Pillared-Layered Magnesium Tetraphosphonate Coordination Polymers: Framework Interconversions and Proton Conductivity Studies" Inorganics 6, no. 3: 96. https://doi.org/10.3390/inorganics6030096
APA StyleColodrero, R. M. P., Salcedo, I. R., Bazaga-García, M., Barouda, E., Papadaki, M., Papathanasiou, K. E., Hernández-Alonso, D., Rius, J., Aranda, M. A. G., Losilla, E. R., Olivera-Pastor, P., Demadis, K. D., & Cabeza, A. (2018). High-Throughput Synthesis of Pillared-Layered Magnesium Tetraphosphonate Coordination Polymers: Framework Interconversions and Proton Conductivity Studies. Inorganics, 6(3), 96. https://doi.org/10.3390/inorganics6030096