Synthesis and Characterization of the New Dicyanamide LiCs2[N(CN)2]3
Abstract
:1. Introduction
2. Results and Discussion
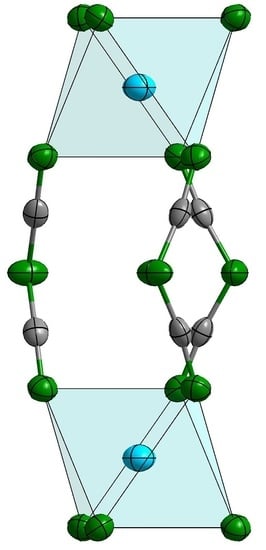
2.1. Structural Description and Discussion
2.2. Vibrational Spectra
3. Materials and Methods
3.1. Synthesis
3.2. Single-Crystal Diffraction
3.3. Infrared and Raman Spectra
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sougrati, M.T.; Darwiche, A.; Liu, X.; Mahmoud, A.; Hermann, R.P.; Jouen, S.; Monconduit, L.; Dronskowski, R.; Stievano, L. Transition-Metal Carbodiimides as Molecular Negative Electrode Materials for Lithium- and Sodium-Ion Batteries with Excellent Cycling Properties. Angew. Chem. Int. Ed. 2016, 55, 5090–5095. [Google Scholar] [CrossRef] [PubMed]
- Sougrati, M.T.; Arayamparambil, J.J.; Liu, X.; Mann, M.; Slabon, A.; Stievano, L.; Dronskowski, R. Carbodiimides as energy materials: Which directions for a reasonable future? Dalton Trans. 2018, 47, 10827–10832. [Google Scholar] [CrossRef] [PubMed]
- Scholz, T.; Görne, A.L.; Dronskowski, R. Itinerant nitrides and salt-like guanidinates—The diversity of solid-state nitrogen chemistry. Prog. Solid State Chem. 2018, 51, 1–18. [Google Scholar] [CrossRef]
- Manson, J.L.; Kmety, C.R.; Huang, Q.-z.; Lynn, J.W.; Bendele, G.M.; Pagola, S.; Stephens, P.W.; Liable-Sands, L.M.; Rheingold, A.L.; Epstein, A.J.; et al. Structure and Magnetic Ordering of MII[N(CN)2]2 (M = Co, Ni). Chem. Mater. 1998, 10, 2552–2560. [Google Scholar] [CrossRef]
- Raebiger, J.W.; Manson, J.L.; Sommer, R.D.; Geiser, U.; Rheingold, A.L.; Miller, J.S. 1-D and 2-D Homoleptic Dicyanamide Structures, [Ph4P]2{CoII[N(CN)2]4} and [Ph4P]{M[N(CN)2]3} (M = Mn, Co). Inorg. Chem. 2001, 40, 2578–2581. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.V.K.; Basaran, A.T.; Ülker, E.; Mishra, R.; Karadas, F. Metal Dicyanamides as Efficient and Robust Water-Oxidation Catalysts. ChemCatChem 2017, 9, 300–307. [Google Scholar] [CrossRef]
- Yoon, H.; Lane, G.H.; Shekibi, Y.; Howlett, P.C.; Forsyth, M.; Best, A.S.; MacFarlane, D.R. Lithium electrochemistry and cycling behaviour of ionic liquids using cyano based anions. Energy Environ. Sci. 2013, 6, 979–986. [Google Scholar] [CrossRef]
- Jürgens, B.; Höppe, H.A.; Irran, E.; Schnick, W. Transformation of Ammonium Dicyanamide into Dicyandiamide in the Solid. Inorg. Chem. 2002, 41, 4849–4851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reckeweg, O.; DiSalvo, F.J.; Schulz, A.; Blaschkowski, B.; Jagiella, S.; Schleid, T. Synthesis, Crystal Structure, and Vibrational Spectra of the Anhydrous Lithium Dicyanamide Li[N(CN)2]. Z. Anorg. Allg. Chem. 2014, 640, 851–855. [Google Scholar] [CrossRef]
- Jürgens, B.; Irran, E.; Schneider, J.; Schnick, W. Trimerization of NaC2N3 to Na3C6N9 in the Solid: Ab Initio Crystal Structure Determination of Two Polymorphs of NaC2N3 and of Na3C6N9 from X-ray Powder Diffractometry. Inorg. Chem. 2000, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Irran, E.; Jürgens, B.; Schnick, W. Trimerization of alkali dicyanamides M[N(CN)2] and formation of tricyanomelaminates M3[C6N9] (M = K, Rb) in the melt: Crystal structure determination of three polymorphs of K[N(CN)2], two of Rb[N(CN)2], and one of K3[C6N9] and Rb3[C6N9] from X-ray powder diffractometry. Chem. Eur. J. 2001, 7, 5372–5381. [Google Scholar] [CrossRef] [PubMed]
- Starynowicz, P. Structure of caesium dicyanamide. Acta Crystallogr. Sect. C 1991, 47, 2198–2199. [Google Scholar] [CrossRef] [Green Version]
- Jürgens, B.; Irran, E.; Schnick, W. Syntheses, Vibrational Spectroscopy, and Crystal Structure Determination from X-Ray Powder Diffraction Data of Alkaline Earth Dicyanamides M[N(CN)2]2 with M = Mg, Ca, Sr, and Ba. J. Solid State Chem. 2001, 157, 241–249. [Google Scholar] [CrossRef]
- Manson, J.L.; Kmety, C.R.; Epstein, A.J.; Miller, J.S. Spontaneous Magnetization in the M[N(CN)2]2 (M = Cr, Mn) Weak Ferromagnets. Inorg. Chem. 1999, 38, 2552–2553. [Google Scholar] [CrossRef]
- Hodgson, S.A.; Hunt, S.J.; Sørensen, T.J.; Thompson, A.L.; Reynolds, E.M.; Faulkner, S.; Goodwin, A.L. Anomalous Thermal Expansion and Luminescence Thermochromism in Silver(I) Dicyanamide. Eur. J. Inorg. Chem. 2016, 2016, 4378–4381. [Google Scholar] [CrossRef]
- Reckeweg, O.; Schulz, A.; Schneck, C.; Lissner, F.; Schleid, T. Syntheses, single-crystal structures, vibrational spectra and DSC/TG analyses of orthorhombic and trigonal Ag[N(CN)2]. Z. Naturforsch. B 2016, 71, 827–834. [Google Scholar] [CrossRef]
- Jürgens, B.; Irran, E.; Höppe, H.A.; Schnick, W. Phase Transition of a Dicyanamide with Rutile-like Structure: Syntheses and Crystal Structures of α- and β-Cd[N(CN)2]2. Z. Anorg. Allg. Chem. 2004, 630, 219–223. [Google Scholar] [CrossRef]
- Reckeweg, O.; Dinnebier, R.E.; Schulz, A.; Blaschkowski, B.; Schneck, C.; Schleid, T. About the air- and water-stable copper(I) dicyanamide: Synthesis, crystal structure, vibrational spectra and DSC/TG analysis of Cu[N(CN)2]. Z. Naturforsch, B 2017, 72, 159–165. [Google Scholar] [CrossRef]
- Manson, J.L.; Lee, D.W.; Rheingold, A.L.; Miller, J.S. Buckled-layered Structure of Zinc Dicyanamide, ZnII[N(CN)2]2. Inorg. Chem. 1998, 37, 5966–5967. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, B.; Irran, E.; Schnick, W. Synthesis and characterization of the rare-earth dicyanamides Ln[N(CN)2]3 with Ln = La, Ce, Pr, Nd, Sm, and Eu. J. Solid State Chem. 2005, 178, 72–78. [Google Scholar] [CrossRef]
- Nag, A.; Schnick, W. Synthesis, Crystal Structure and Thermal Behavior of Gadolinium Dicyanamide Dihydrate Gd[N(CN)2]3·2H2O. Z. Anorg. Allg. Chem. 2006, 632, 609–614. [Google Scholar] [CrossRef]
- Nag, A.; Schmidt, P.J.; Schnick, W. Synthesis and Characterization of Tb[N(CN)2]3·2H2O and Eu[N(CN)2]3·2H2O: Two New Luminescent Rare-Earth Dicyanamides. Chem. Mater. 2006, 18, 5738–5745. [Google Scholar] [CrossRef]
- Reckeweg, O.; Wakabayashi, R.H.; DiSalvo, F.J.; Schulz, A.; Schneck, C.; Schleid, T. About alkali metal dicyanamides: Syntheses, single-crystal structure determination, DSC/TG and vibrational spectra of KCs[N(CN)2]2 and NaRb2[N(CN)2]3·H2O. Z. Naturforsch. B 2015, 70, 365–372. [Google Scholar] [CrossRef]
- Reckeweg, O.; DiSalvo, F.J. Synthesis and single-crystal structure of the pseudo-ternary compounds LiA[N(CN)2]2 (A = K or Rb). Z. Naturforsch. B 2016, 71, 157–160. [Google Scholar] [CrossRef]
- Jürgens, B.; Milius, W.; Morys, P.; Schnick, W. Trimerisierung von Dicyanamid-Ionen C2N3—im Festkörper—Synthesen, Kristallstrukturen und Eigenschaften von NaCs2(C2N3)3 und Na3C6N9·3H2O. Z. Anorg. Allg. Chem. 1998, 624, 91–97. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. Sect. B 1991, 47, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Bruker AXS Inc. SAINT Version 7.68A; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Bruker AXS Inc. SADABS Version 2004/1; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]



| LiCs2[dca]3 | NaCs2[dca]3 [25] | ||
|---|---|---|---|
| Li–N1 (6×) | 2.2823(1) | Na–N1 (6×) | 2.468(3) |
| Cs–N2 (3×) | 3.5225(2) | Cs–N2 (3×) | 3.629(3) |
| Cs–N1 (3×) | 3.2289(2) | Cs–N1 (3×) | 3.284(3) |
| Cs–N1 (3×) | 3.2473(2) | Cs–N1 (3×) | 3.276(3) |
| Cs–N1 (3×) | 3.741(2) | ||
| N1–C | 1.1647(1) | N1–C | 1.154(4) |
| N2–C (2×) | 1.3133(1) | N2–C (2×) | 1.315(3) |
| ∡(C–N2–C) | 119.792(7) | ∡(C–N2–C) | 119.5(2) |
| ∡(N1–C–N2) | 172.140(4) | ∡(N1–C–N2) | 172.6(3) |
| νIR(LiCs2[dca]3) | νRaman(LiCs2[dca]3) | ν(NaCs2[dca]3) [25] | ||||
|---|---|---|---|---|---|---|
| σas(N–C≡N) | 515 | - | 516 | |||
| γas(N–C≡N) | - | 522 | 526 | |||
| γs(N–C≡N) | - | 545 | 543 | |||
| σs(N–C≡N) | 663 | 669 | 666 | |||
| νs(N–C) | 913 | 920 | 930/917 | |||
| νas(N–C) | 1333 | - | 1342 | |||
| νs(N–C) + σs(N–C≡N) | 1428 | - | 1437 | |||
| νas(N≡C) | 2146 | 2133 | 2167 | |||
| νas(N–C) + νs(N–C) | 2203 | 2218 | 2228/2206 | |||
| νs(N≡C) | 2265 | - | 2286/2267 | |||
| νas(N≡C) + νs(N–C) | 3036 | - | 3061 | |||
| νs(N≡C) + νs(N–C) | 3178 | - | 3213/3179 | |||
| νs(N≡C) + νas(N–C) | 3529 | - | 3549/3528/3471 |
| Chemical Formula | LiCs2[dca]3 |
|---|---|
| Formula weight (g∙mol−1) | 434.85 |
| Crystal system | hexagonal |
| Space group | P63/m (no. 176) |
| Temperature (K) | 100 |
| a (Å) | 6.8480(8) |
| c (Å) | 14.1665(17) |
| V (Å3) | 575.33(1) |
| Z | 2 |
| Radiation, λ (Å) | Mo-Kα1, 0.71073 |
| μ (mm−1) | 6.317 |
| Crystal shape and color | Colorless block |
| Crystal size (mm3) | 0.02 × 0.02 × 0.03 |
| ρcalc (g∙cm−3) | 2.7181 |
| Diffractometer | Bruker APEX CCD |
| Absorption correction | Multi-scan, SADABS 2014/15 |
| Tmin, Tmax | 0.5361, 0.7461 |
| No. of measured, independent and observed [I>3σ(I)] reflections | 4655, 617, 528 |
| Robs | 0.014 |
| Rall | 0.018 |
| GOFobs | 1.07 |
| No. of parameters, restraints | 30, 0 |
| Atom | Site | x | y | z | Ueq |
|---|---|---|---|---|---|
| Li | 2b | 0 | 0 | 0 | 0.0144(17) |
| Cs | 4f | ⅓ | ⅔ | 0.078375(12) | 0.00920(6) |
| N2 | 6h | 0.3590(4) | 0.3077(4) | ¼ | 0.0155(9) |
| N1 | 12i | 0.3115(2) | 0.1284(2) | 0.09385(11) | 0.0122(6) |
| C | 12i | 0.3333(3) | 0.2014(3) | 0.16978(12) | 0.0098(6) |
| Atom | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|
| Li | 0.016(2) | 0.016(2) | 0.010(3) | 0.0082(10) | 0 | 0 |
| Cs | 0.00892(8) | 0.00892(8) | 0.00975(10) | 0.00446(4) | 0 | 0 |
| N2 | 0.0245(11) | 0.0113(10) | 0.0101(9) | 0.0084(10) | 0 | 0 |
| N1 | 0.0134(7) | 0.0122(7) | 0.0107(7) | 0.0062(6) | 0.0005(5) | 0.0017(5) |
| C | 0.0089(7) | 0.0086(7) | 0.0128(8) | 0.0050(6) | 0.0006(6) | 0.0033(6) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mann, M.; Reckeweg, O.; Dronskowski, R. Synthesis and Characterization of the New Dicyanamide LiCs2[N(CN)2]3. Inorganics 2018, 6, 108. https://doi.org/10.3390/inorganics6040108
Mann M, Reckeweg O, Dronskowski R. Synthesis and Characterization of the New Dicyanamide LiCs2[N(CN)2]3. Inorganics. 2018; 6(4):108. https://doi.org/10.3390/inorganics6040108
Chicago/Turabian StyleMann, Markus, Olaf Reckeweg, and Richard Dronskowski. 2018. "Synthesis and Characterization of the New Dicyanamide LiCs2[N(CN)2]3" Inorganics 6, no. 4: 108. https://doi.org/10.3390/inorganics6040108
APA StyleMann, M., Reckeweg, O., & Dronskowski, R. (2018). Synthesis and Characterization of the New Dicyanamide LiCs2[N(CN)2]3. Inorganics, 6(4), 108. https://doi.org/10.3390/inorganics6040108