1. Introduction
Molecular imaging offers the capacity of detecting and following-up events of processes happening at cellular and molecular levels that are signature (biomarkers) of early stages of a disease. Such medical exams require the use of a contrast agent (CA) that is specific to the event or process to detect, or which signal depends on the later.
Molecular magnetic resonance imaging (MRI) is a powerful non-invasive imaging technique which takes advantage of a very high spatial and temporal resolution and can provide detailed molecular/cellular information when combined with a contrast agent (to over-cross the lack of sensitivity inherent to MRI, see below). The MRI CA are molecules or particles that influence the relaxation of water protons. They can be T1-(positive) or T2-(negative) weighted, affecting the longitudinal or transverse relaxation times respectively. T1-weighted CA are mostly small gadolinium (Gd3+) or manganese (Mn2+) paramagnetic complexes, while the majority of the T2-weighted ones are iron oxide-based superparamagnetic nanoparticles. This review will focus on Gd3+-based small molecule contrast agents.
MRI is widely used in clinics, with more than 30% of the exams made with the help of a CA, since the late 80’s. Mostly all commercially available T
1-based MRI contrast agents are based on Gd
3+ complexes of DTPA (diethylenetriamine pentaacetic acid) or DOTA (1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid) derivatives. Nevertheless, amongst these, only one is specific: gadofosveset (
Vasovist®;
Alblatar®), used for the targeting of human serum albumin.
Figure 1a shows the chemical structure of four Gd
3+ complexes in clinical use,
Dotarem® (DOTA-based),
Magnevist® (DTPA-based),
ProHance® (HP-DO3A, 2,2′,2″-[10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl]triacetate) and
Vasovist® (DTPA-based).
Several parameters govern the paramagnetic enhancement efficacy of a CA (or relaxivity,
r1, expressed per millimolar concentration of Gd
3+, in mM
−1·s
−1) but the main ones that can be tuned easily by the chemists while designing novel and more efficient CAs are: (1) the number of water molecules directly bound to the Gd
3+ ion,
q (hydration number); (2) the water exchange rate of these water molecules with those of the bulk,
kex and (3) the rotational correlation time of the complex,
τR [
1]. A schematic representation of these parameters is depicted in
Figure 1b.
The CAs in clinical use are low molecular weight Gd3+-based complexes (0.5–1 KDa) with one water molecule in the inner-sphere, which relaxivities range from 3 to 5 mM−1·s−1 at 1.5 or 3 T (clinical magnetic fields). When administered, these imaging probes will distribute within the organism without a specific target (except for Vasovist®) and are able to give information on pathologies that result in changes in the vascular volume, perfusion and permeability of the tissues/organs. Such alterations occur typically in more advanced stages of disease. The use of targeted probes that are able to highlight modifications at a molecular/cellular level, before any clinical phenotype of disease is observable, is highly desired.
Even though the design of novel targeted probes is an active research field, the majority of the imaging probes reported in the literature is not in clinical application but still in preclinical validation phase, mostly in phase I or II clinical trials. Vasovist® has seen its production coming to an end due to insufficient sale numbers, while for example Gd-ESMA, an elastin-specific contrast agent, from Lantheus Medical Imaging (see below) is their pipeline in late-stage preclinical studies. There is a clear need for more translational efforts.
Overcoming the low MRI sensitivity
Due to the lack of sensitivity of MRI, relatively high concentrations of CA (mM range) need to be injected to generate local variation in the water signal intensity and, therefore, detectable signal.
Typical biomarkers include receptors or proteins which are overexpressed in case of disease. These are present in vivo usually in low concentrations, often in nM scale. The formation of protein “microdomains” can occur, due to different conformation of a protein and could lead to higher “retention” of a CA and thus to its higher local concentrations. By consequence, a higher local signal enhancement can occur. Estimations based on the parameters of the clinical CA available and the recommended injected dose for an MRI probe (0.1 mmol Gd
3+/kg), showed that to get a detectable signal enhancement, a minimum of 100 µM Gd
3+ local concentration is needed. In case of “microdomain” this detection limit can be up to 2.5-fold lower [
2,
3].
Signal amplification strategies are then required to achieve good images, enabling the assessment of the desired biomarkers. Among the reported strategies are: accumulation of a large number of Gd3+ at the site of interest, by using macromolecular systems (e.g., polymers or nanoparticles); and the use of targeted probes, which have their efficacy changing when binding to their biomarker. The rational design of novel generation of CA is in general accompanied by the optimization of the stability and relaxivity features of the Gd3+ complex unit.
2. Rational Design of Targeted Contrast Agents
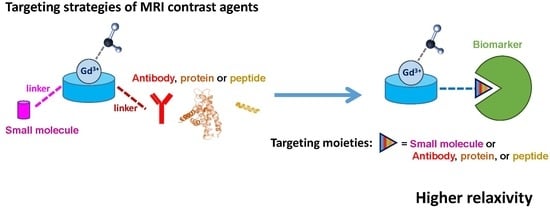
Different targeting moieties can be used to achieve a specific binding to the chosen biomarker.
Figure 2 summarizes the type of moieties potentially used: in general, the probes are based on a chelating unit for the Gd
3+ (aiming to guarantee a good kinetic inertness and thermodynamic stability of the metal complex but also the presence of at least one water molecule in the inner-sphere), which is conjugated to a small molecule, antibody, protein or peptide via a linker (its length and type can vary, ensuring that the good affinity towards the target is not affected by the presence of the metal complex). When bound to a highly concentrated biomarker in vivo (e.g., human serum albumin), the complex “MRI probe–biomarker” has a higher rotational correlation time, yielding higher relaxivity and hence a higher enhanced signal. If the biomarker is a receptor (lower in vivo concentrations), the signal enhancement results mostly from the high local concentration of the CA (multimeric-like effect).
Other strategies include the development of macromolecules (usually nanoparticles, the most explored ones being liposomes) that incorporate, within the same unit, a high payload of Gd
3+ ions and a targeting moiety. Gd
3+ containing nanoparticle based CA have been revised elsewhere [
4,
5,
6,
7,
8].
T
2-weigthed based contrast agents for cell tracking or specific targeting have also been developed [
9,
10,
11].
2.1. Pharmacokinetics, Specificity and Affinity of a Contrast Agent
The biomarkers generally chosen as MRI targets (in general cell surface receptors or extracellular proteins) do not require the contrast agent being able to penetrate the tissue/organ and leave the extravascular compartment. The biodistribution properties of a CA are highly dependent on its size, shape and charge. Small-molecular CA injected intravenously are in general rapidly distributed in the vascular system and eliminated through the kidneys, where particles below 5 nm are filtered regardless of their charge. Larger molecules can undergo adsorption or opsonization by serum proteins and can see their size altered: the in vivo hydrodynamic diameter is typically higher than their in vitro one. This affects their blood clearance and half-life. These larger molecules are eliminated in the kidneys depending on their global charge and size. Macromolecules or particles larger than 8 nm do not undergo glomerular filtration and are excreted through the hepatobiliary system [
12,
13,
14]. Such aspects can be considered when conceiving a novel probe, depending on the aimed biodistribution and half-life.
The affinity and specificity towards the target when designing a targeted CA is a major concern. It is desired to achieve a good specificity in order to guarantee that the signal observed is due to the binding towards the protein/tissue of interest. It is also important to have a good reversible affinity, within the time-scale of the imaging protocol. The large majority of the reported targeted-CAs have affinities in the nM to µM range, which seems to be sufficient to perform an MRI, while avoiding the triggering of an immune response from the body, due to the presence of the CA.
2.2. Small Molecules versus Antibody, Protein or Peptide
Compared to their protein-based counterpart probes, small molecule-based and peptide-based probes present a more straightforward chemical and relaxometric characterization and are more easily tuned in terms of improving their in vivo stability.
The use of small peptides instead of small molecules to target the same biomarker usually leads to stronger binding affinity and selectivity. Moreover, small peptides are in general promising targeting units when compared with full proteins, mainly due to their appealing properties such as low immunogenicity leading to high biocompatibility, more resistant to in vivo enzymatic degradation, favourable pharmacokinetics with fast excretion, good tissue distribution and fast clearance from blood. Peptide structure modifications are often used to adjust their stability and half-life. These include the use of D-amino acid residues, cyclization and replacement of peptide bonds with amide bond surrogates.
On the other hand, the use of an antagonist peptide enables better target uptake with comparable binding affinity and no active internalization, compared to agonist peptides. These differences are mostly due to a higher number of binding sites of antagonists and their slower dissociation from the receptor. Antagonist peptides are also more resistant to cell membrane enzymes, which might also contribute to the higher uptake of antagonist peptides [
15,
16].
3. Different Targets Moieties for the Same Biomarker: Selected Examples
Serum albumin is a highly available plasma protein and has been the biomarker of choice for blood pool contrast agents. Extracellular matrix (ECM) proteins are also vastly explored as biomarkers for several diseases, namely for different types of cancer, metastasis or cardiovascular diseases (CVD) [
17,
18].
Below are selected examples of MRI targeted probes for serum albumin, ECM proteins (elastin, tropoelastin, collagen, fibrin and fibronectin), epidermal growth factor receptor, protein tyrosine phosphatase mu and amyloid aggregated-like proteins.
3.1. Human Serum Albumin (HSA)
Human serum albumin (HSA) is the protein present in higher concentration in the blood (≈0.6 mM). Several research groups have developed blood pool probes which target HSA, based on different targeting approaches. From the reported probes,
Vasovist® (
Figure 1a) has reached clinical application but its production has been discontinued in 2017 due to poor sales.
The structure of
Vasovist® is based on a biphenycyclohexyl moiety, conjugated to a
Gd-DTPA unit via a phosphodiester linker, which binds HSA with an affinity of 100 µM. This probe has a relaxivity of 6.6 mM
−1·s
−1 in its “free form”, while in the presence of HSA containing human plasma it reaches 42 mM
−1·s
−1, at 0.47 T [
19,
20]. Indeed, the rotational correlation time is the key parameter which accounts for this relaxivity increase:
τR of
Gd-DTPA is 58 ps, while for the “complex”
Vasovist-HSA it is 3000–4000 ps [
1].
HSA-probes based on different chelate scaffolds have also been developed.
Gd-AAZTA (6-amino-6-methylperhydro-1,4-diazepinetetraacetic acid;
Scheme 1) complex has two water molecules in the coordination sphere of Gd
3+ and displays a relaxivity of 7.1 mM
−1·s
−1 (0.47 T, 25 °C) and a
τR of 74 ps [
21].
The use of this scaffold as chelating unit, conjugated to different targeting moieties has led to higher relaxivities of the targeted “free” probes and of their bound form. For example, the AAZTA-based
Gd-B25716/1 [
22] and the DTPA chelates
Gd-B22956/1 (in phase I trials) [
23,
24] and the very recently reported
(Gd-DTPA)2-Chol [
25], share the same targeting moiety, an analogue of deoxycholic acid (
Scheme 1). The use of the AAZTA chelate unit instead of the DTPA brings an extra water molecule to the inner sphere (
q = 2 for
Gd-AAZTA), which justifies the higher relaxivity of the complex
Gd-B25716/1 in PBS (at 0.47 T): indeed the
r1 is 13.0 mM
−1·s
−1 for
Gd-B25716/1, 1.5-fold higher when compared with 8.6 mM
−1·s
−1 for
B22956/1. Both AAZTA- and DTPA-based complexes bind to albumin with similar affinities but the relaxivity of the HSA-bound forms is 29.0 mM
−1·s
−1 and 26.8 mM
−1·s
−1, respectively. In fact, when the AAZTA-based complex binds to HSA the loss of one water molecule from the inner sphere is observed but the expected decrease in relaxivity for the global monohydrated “
HSA-(Gd-B25716/1)” complex is somehow compensated by the contribution of second-sphere water molecules present and thus presenting a similar relaxivity than “
HSA-(Gd-B22956/1).”
Longo et al. reported on an AAZTA-based CA,
Gd-AAZTA-MADEC (
Scheme 1), conjugated to a deoxycholic acid moiety through a longer spacer, with a 9 CH
2 chain, instead of 2 for
Gd-B25716/1. The use of a longer linker results in a higher affinity towards HSA (≈1 µM), about 100-fold higher compared to the aforementioned CA. This improved affinity, together with optimal pharmacokinetic and relaxometric properties (blood half-life of 32.6 min;
r1 of 13.9 mM
−1·s
−1 in its “free form” and 38.7 mM
−1·s
−1 in the presence of HSA (0.47 T)) make this Gd-based complex a good blood pool CA (
Figure 3).
Gd-AAZTA-MADEC has been tested in vivo, showing significant enhancement of the vasculature and extravasation inside tumour for a prolonged period of time [
26].
The recently reported dinuclear Gd
3+ complex
(Gd-DTPA)2-Chol has a relaxivity of 7.7 mM
−1·s
−1 in saline and 21.2 mM
−1·s
−1 in presence of HSA. The use of a dimer and thus its increase in molecular weight did not lead to a high gain of relaxivity. With similar in vivo “bond” relaxivities for
Gd-B22956/1 and
(Gd-DTPA)2-Chol as well as their HSA affinity (≈125 µM),
(Gd-DTPA)2-Chol stands out for its longer blood half-life of 128 min, about 7-fold higher than the half-lives of
Gd-B22956/1 (17.5 min [
25]),
Vasovist® (23 min [
20]) or
Gd-DTPA (17.7 min [
25]).
3.2. Elastin/Tropoelastin
Elastin is the most abundant protein in the ECM. Dysfunctional ECM contributes to the initiation, progression and complication of arterial diseases, including abdominal aortic aneurysm and atherosclerosis. Elastin in its mature form is an insoluble fibre, consisted of tropoelastin soluble monomers crosslinked by the enzyme Lysil Oxidase (LOX). Tropoelastin is typically produced and converted into elastin in the late fetal and early neonatal periods. Normal arteries contain mature elastin but insignificant amounts of tropoelastin, whose production is resumed in case of disease [
17,
27].
Elastin has been explored as a biomarker for cardiovascular diseases (CVD) and an MRI contrast agent
Gd-ESMA (
Scheme 2) was described by Onthank et al., which is now in late-stage preclinical studies led by Lantheus Medical Imaging [
28,
29]. This CA was validated in animal models of different CVD, such as atherosclerosis [
28], aortic abdominal aneurysm [
30] and myocardial infarction [
31]. Its structure was primarily based on a DTPA chelate, later on developed as a DOTA derivative, conjugated to a single amino acid
D-phenylalanine. The relaxivity of
Gd-ESMA is 4.5 mM
−1·s
−1 in its “free” form and 13.7 mM
−1·s
−1 when bound to elastin, at 1.5 T [
28]. Nevertheless, this compound binds equally to both elastin and tropoelastin (40% binding), with an affinity (
KD) of 1 ± 0.5 µM for elastin and 9.2 ± 0.7 µM for tropoelastin [
30].
Aiming for a better discrimination of elastin and tropoelastin, Phinikaridou et al. have developed a specific CA for tropoelastin. This novel CA consists of a DOTA chelate conjugated to a 15 amino acid peptide:
(Gd-DOTA)-VVGSPSAQDEASPLS or
Gd-TESMA (
Scheme 2) and allows selective targeting of the monomer (tropoelastin) versus the fibre form (elastin) [
32].
The targeting moiety peptide has been selected from the epitope of binding of the elastin-binding protein (EBP) to tropoelastin. EBP is a subunit of the elastin/laminin receptor which is responsible for chaperoning the secreted tropoelastin to the extracellular space.
A good discrimination in the binding to tropoelastin vs elastin was achieved with this new probe: 64 ± 7% versus 1 ± 2% (p = 0.001), respectively, although its affinity remains in the same range as Gd-ESMA (KD of 2.37 ± 0.05 µM for tropoelastin and of 14 ± 5 µM for elastin). This new CA has a high in vitro relaxivity in solution (11.7 ± 0.6 mM−1·s−1) and presents a relaxivity of 44 ± 1 mM−1·s−1 when bound to tropoelastin in vitro (0.47 T). In vivo studies on a murine model of atherosclerosis revealed a lower in vivo relaxivity value which drops to 7.15 mM−1·s−1 (3 T). This difference was attributed to the different (higher) magnetic field and to the fact that some of the binding sites of tropoelastin might be masked in vivo, together with the less accessibility of water within the atherosclerotic lesion.
For this system, the use of a small peptide instead of a single amino acid provided a good in vitro and in vivo discrimination of the monomer versus the fibre and therefore enabling discrimination of diseased and “normal” vessel wall.
Figure 4 shows the in vivo MRI comparison of vessel wall enhancement using the elastin (
Gd-ESMA, 0.2 mmol Gd
3+/kg) and tropoelastin (
Gd-TESMA, 0.2 mmol Gd
3+/kg) CAs in control and diseased mice (atherosclerotic apolipoprotein E–deficient murine model (ApoE
−/−)). The figure displays the fused maximum intensity projection reconstructed magnetic resonance angiography and delayed-enhanced-MRI after administration of both CA to the same animal scanned 24 h apart, showing focal uptake in the brachiocephalic artery (BCA). The MRI of the BCA acquired from a control animal showed vessel wall uptake of
Gd-ESMA (panels
Figure 4A,B) but no uptake of
Gd-TESMA (panels
Figure 4C,D) due to the lack of tropoelastin in the absence of disease. Nevertheless, MRI of the BCA acquired from a diseased animal showed enhancement of the vessel wall after administration of both agents because of the presence of both cross-linked elastin and tropoelastin in the atherosclerotic lesion. The discrimination of normal versus diseased BCAs is clearer when using
Gd-TESMA.
3.3. Collagen
There are 28 different types of collagen, mostly present at the ECM as triple-stranded fibres, which can further interact to form networks. They confer tensile strength, regulate cell adhesion, promote cell migration and are also involved in chemotaxis. Imbalance of collagen expression or ratio between types of collagen are associated with several diseases, including CDV and cancer, making them potential disease biomarkers [
18].
Several imaging probes have been explored, for example, based on phage display and amino acid mutation studies Caravan et al. were able to identify a collagen binding peptide: GKWHCTTKFPHHYCLY [
33]. This peptide has then been conjugated to three Gd-DTPA units and termed
EP-3533 (
Scheme 3). This probe has good affinity for type I collagen (
KD = 1.8 ± 1.0 µM) and a relaxivity of 16.1 mM
−1·s
−1 per Gd
3+ (or 48.3 mM
−1·s
−1 per molecule) at 1.4 T and has enabled visualization of fibrotic scar versus viable myocardium and blood in a mouse model of chronic infarction [
34].
3.4. Fibrin and Fibronectin
Fibrin is the major component of the blood clot. It is involved in wound healing and tissue repair and is implicated in processes of adhesion, proliferation and migration of cells involved in the repairing process. Changes in fibrin expression, or its degradation and organization were associated with a number of pathologies, including thrombosis, infection, inflammation, fibrosis and cancer. Fibronectin is a glycoprotein which contains two major fibrin-binding sites and is enzymatically converted in fibrin. It mediates a large variety of cellular interactions with the ECM and plays an important role in cell adhesion, migration, growth and differentiation [
17,
18].
3.4.1. Fibrin
Fibrin has been largely explored as imaging target. Amongst the specific contrast agents developed, the majority is based on small peptide moieties. For example, Caravan et al. developed several CAs based on small peptides anchored on DTPA or DOTA chelates and bearing one to four units per molecule, namely,
EP-1873 (DTPA-based) [
35] and
EP-2104R (DOTA-GA-based;
Scheme 4) [
36] probes containing the peptide (X
nCPYGLCX
m). The chosen peptides bind to fibrin with
KD in the range 4 to 9 μM and have a remarkable selectivity for fibrin over fibronectin (100-fold). Both complexes have 4 Gd
3+ units and present relaxivities of 84 mM
−1·s
−1 (21 mM
−1·s
−1 per Gd
3+) and 71.4 mM
−1·s
−1 (17.4 mM
−1·s
−1 per Gd
3+) at 1.4 T, respectively, when bound to fibrin.
EP-2104R allowed detection of thrombus in a rat ischemic stroke model.
Figure 5 shows a coronal slice from middle cerebral artery 3D T1-weighted images of the detection of an intracranial thrombus using
EP-2104R. This probe has reached phase II trials and enabled visualization of 52 patients with previously diagnosed with thrombi in the venous, heart or arterial system [
37,
38].
Earlier, Lu et al. have explored the cyclic CLT1 peptide (CGLIIQKNEC) conjugated to a DTPA unit
(Gd-DTPA)-CLT1,
Scheme 4), which binds specifically to the complex fibrin-fibronectin. This CA presents a relaxivity of 4.2 mM
−1·s
−1 at 3 T and was evaluated in athymic nu/nu mice bearing HT-29 human colon carcinoma xenografts. A good enhancement was observed in the tumour. Due to the involvement of fibrin-fibronectin complexes in several pathologies, the authors claim that
(Gd-DTPA)-CLT1 has a great potential for cancer detection, as well as diagnosis, characterization of tumour angiogenesis and imaging of wounds and atherosclerosis [
39].
Aime et al. followed a similar approach to that of Caravan and co-workers, developing an AAZTA-tetramer conjugated to the same fibrin-binding peptide used for
EP-2104R (X
nCPYGLCX
m) [
40]. This novel probe—
FibPep-(Gd-AAZTA)4 (
Scheme 5)—relies on four Gd-AAZTA(CH
2)
4COOH units, which are coupled to a trilysine scaffold, functionalized with a maleimide function, which is then conjugated to the cyclic 11-amino acid fibrin-binding peptide (FibPep), previously reported by Caravan et al. [
36].
This novel probe has a relaxivity of 18.5 ± 0.3 mM
−1·s
−1 per Gd
3+ or 74 ± 1 mM
−1·s
−1 per molecule in water; of 22.5 ± 0.2 mM
−1·s
−1 per Gd
3+ in serum and higher relaxivity of 58 ± 13 mM
−1·s
−1 per Gd
3+ in presence of fibrin plasma clots (0.5 T). The binding profile of this CA to fibrin shows specific and unspecific binding regions depending on the concentration range. An affinity (
KD) of 3.0 ± 0.6 µM has been determined by fitting the data for the higher affinity site (up to 10 µM) [
40].
3.4.2. Extradomain B Fibronectin
Prostate cancer still accounts for 30% death of patients, making earlier and more accurate detection of the disease an unmet clinical need. Lu and co-workers have explored four novel MRI contrast agents that target extradomain-B fibronectin (EDB-FN; overexpressed in the ECM of aggressive tumours but absent in healthy tissue), as cancer biomarkers [
41,
42,
43]. These recent probes are based on HP-DO3A (
Figure 1a) or DOTA derivatives conjugated to small peptides which target EDB-FN, via a short PEG linker. The peptides have been identified by phage display and show strong affinity towards human EDB-FN. The first peptide studied is a 7 amino acid residue named ZD2 (TVRTSAD) and has been conjugated to both HP-DO3A [
43] and DOTA [
42] chelates.
ZD2-(Gd(HP-DO3A)) (
KD of 1.7 µM) has a relaxivity of 5.4 mM
−1·s
−1 while the one of the analogue
ZD2-(Gd-DOTA) is 4.1 mM
−1·s
−1 at 1.5 T [
43].
Another three sequences were also identified and have been conjugated to DOTA derivative, via reaction with
p-NCS-Bz-DOTA-GA (2,2′,2″-(10-(1-carboxy-4-((4-isothiocyanatobenzyl)amino)-4-oxobutyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid) which results in an additional isothiocyanate group in the linker:
GVK-(Gd-DOTA), GVK = GVKSYNE;
IGK-(Gd-DOTA), IGK = IGKTNTL and
SGV-(Gd-DOTA), SGV = SGVKSAF [
41].
In silico simulations with the peptides showed their binding patterns towards the EDB-FN (
Figure 6), revealing that these peptides bind to different sites of the EDB fragment.
All compounds have been tested in vivo by MRI in PC3 tumour bearing mice, showing tumour uptake and good signal enhancement compared with the non-specific counterparts Gd-DOTA or Gd-(HP-DO3A). The tumour affinity and specificity were confirmed by studying the in vivo and ex vivo fluorescence of the Cy5.5-peptide derivatives. These additional three probes have similar relaxivities at 1.5 T (4.3 to 4.7 mM−1·s−1) but the biodistribution studies revealed a stronger binding for IGK peptide that is similar to ZD2.
3.5. Epidermal Growth Factor Receptor/HER2 (EGFR/HER2)
The HER2 epidermal growth factor receptors (EGFR/HER2) are overexpressed in various types of cancer cells and constitute a known biomarker for cancer prognosis. These are already in clinical use as biomarkers, namely for breast cancer but their quantification relies on invasive methods such as biopsy followed by measurement of the proteins by molecular biology techniques and in general lead to inaccurate results. Non-invasive detection of the higher levels of these proteins directly in vivo would simplify the detection and allow treatment monitoring of early stage breast cancer tumours.
Yang et al. have reported on the development of novel type of Gd
3+-binding protein MRI probes, ProCA [
3,
44,
45]. The scaffold of these CAs is based on the domain 1 of CD2. This protein is expressed at the surface of natural killer cells and T cells and is implicated in signal transduction and cell adhesion. Its attractive features as scaffold include the rigidity, resistance to pH changes and enzyme cleavage and high tolerance for mutations.
The stable coordination of Gd
3+ is assured by the presence of five Aspartic acid (Asp or D) and Glutamic acid (Glu or E) carboxylic side chains or by incorporating unnatural amino acids into the sequence, whose coordination sphere is completed by two water molecules. The binding pocket design takes into account the good exposition of Gd
3+ towards the bulk water, enabling an exchange to happen, essential for MRI. This coordination design has been optimized by computer simulations (
Figure 7) and enabled the design of different CA’s. The first generation, ProCA1, has a relaxivity of 117 mM
−1·s
−1 at 1.5 T and 48 mM
−1·s
−1 at 3 T (medical fields) and a
q of 2. The thermodynamic stability of these complexes is relatively low (log
KML ≈ 12.1 [
44]; while log
KML(Gd-DTPA) ≈ 22.46 [
1]) but improved stability has been achieved for the latest generation ProCA3, displaying a log
KML of 21.6 [
46]. Nonetheless, this first generation showed good stability (no transmetallation) when tested under conditions simulating the extracellular environment and no effect of other biological metal ions on the relaxivities of ProCAs was observed [
46]. Further improvement of this generation of CA included its PEGylation for a better blood half-life, which also resulted in an increase of 30–100% in their relaxivity.
Moreover, specific ProCA-based CAs targeting HER2 (ProCA1.affi). The targeted CAs consist on the conjugation of ProCA1 to a targeted moiety using antibodies or affibodies, either by conjugation via a flexible linker to the C-terminus of the Gd
3+-binding protein or by a biotin-avidin method [
3,
45]. The successful targeting has been validated in vivo in nude mice xenograft models of SKOV3 and MDA-MB-231 human cell lines for HER2. Structure improvements allowed the use of 60- to 100-fold lower dose injections when compared to the clinical doses [
3,
44,
45].
The same authors also reported on other ProCA1-based targeting probes, for example ProCA1.GRPC was proposed as gastrin-releasing peptide receptor probe and tested in PC-3 tumour xenograft mice [
3,
46].
3.6. Protein Tyrosine Phosphatase Mu (PTPµ)
Glioblastoma multiforme (GBM) is a highly lethal brain tumour characterized by its fast proliferation and ability to spread. Normal brain astrocytomas express the receptor protein tyrosine phosphatase mu (PTPµ) in normal conditions, while GBM downregulates the full length expression of this protein. One of the resulting fragments remains associated with GBM tissue setting an interesting biomarker for these tumours. Brady-Kalmay and co-workers have studied MRI probes that target PTPµ based on four peptides SBK1-4, designed from the epitopes of interaction of PTPµ with cell adhesion molecules of µ (SBK1 and 2) or immunoglobulin (SBK3 and 4) domains. From these, SBK2 and SBK4 showed to bind better to PTPµ [
47] and the same group reported on the development of an MRI probe based on the peptide SBK2 (GEGDDFNWEQVNTLTKPTSD) conjugated to 3 Gd-DOTA units via a tris-propargyl linker,
SBK2-Tris-(Gd-DOTA)3 [
48]. This probe has a relaxivity of 8.3 mM
−1·s
−1 per Gd
3+ at 1.41 T and is able to target heterotopic xenograft model of brain tumour, showing prolonged tumour retention when compared with a non-specific CA and a SBK2-scrambled-(Gd-DOTA)
3 version. More recently, a single Gd
3+ unit version of this CA, using a lysine residue as spacer,
SBK2-Lys-(Gd-DOTA), showed a relaxivity of 8.4 ± 0.1 mM
−1·s
−1 at 1.41 T (6.0 ± 0.1 mM
−1·s
−1 at 9.4 T) allowing a good tumour enhancement, in both heterotopic and orthotopic glioma murine models [
49]. Importantly, this recent “mono-Gd
3+” version could be injected in the typical dose (0.1 mmol Gd
3+/kg), while the tris-DOTA version could only be detected using 0.2 mmol Gd
3+/kg.
3.7. Misfolded Amyloid Aggregated-Like Proteins
Aggregation of misfolded proteins is a common hallmark of highly debilitating diseases such as amyotrophic lateral sclerosis, type II diabetes and Parkinson’s and Alzheimer’s diseases. The development of novel non-invasive probes which target misfolded proteins at the origin of these diseases, such as superoxide dismutase, human amylin, α-Synuclein, hyperphosphorylated tau protein and in particular Amyloid-β for Alzheimer’s disease, have been widely explored and reviewed [
50]. The majority of the probes is based on Gd
3+-complexes linked to small molecules, peptides or antibodies. Small molecule-based CAs are mostly based on derivatives of: (1) Pittsburgh compound B (PiB), a derivative of the thioflavin T which binds to fibrillar Aβ amyloid deposits in situ; (2) Curcumin; (3) Congo Red and (4) stilbene. All structures are depicted in
Scheme 6.
Geraldes, Tóth and co-workers explored a series of DOTA-based complexes bearing a modified PiB unit conjugated via different linkers (different length and chemical nature, leading to different charge and lipophilicity of the probes;
Scheme 7) [
51]. These CAs have relaxivities of ≈6 mM
−1·s
−1 in water and ≈14 mM
−1·s
−1 in the presence of aggregated Aβ protein (0.47 T) and showed affinities towards Aβ
1–40 within the 70–200 µM range.
Importantly, radiolabelled analogues (
111In [
52],
68Ga [
53]) of the probes showed promising brain uptake in vivo, both in wild-type and Alzheimer’s disease mice models.
Indeed, the biggest challenge of the probes developed so far relies on the difficulty of blood-brain barrier crossing by the final complexes.
4. Concluding Remarks
In this review the advantages and challenges of the development of targeted molecular imaging probes are presented through selected examples. The main strategy to achieve a targeted CA relies on the optimization of each component, for example, the Gd3+ chelate unit, responsible for the imaging and the targeting moiety, responsible for the recognition of the desired biomarker. Several chelate scaffolds were reported and different conjugation strategies described.
Nevertheless, several parameters rule the final in vivo application of such contrast agents, making their translation to routine use in clinic application a demanding process. Such parameters include: the thermodynamic stability and kinetic inertness of the Gd3+ complex; its conjugation to a targeting unit (which should insure the maintenance of a good affinity and selectivity towards the desired target/biomarker); maximal relaxivity; optimal pharmacokinetic properties and good clearance, while guarantying an adequate imaging window; no in vivo toxicity and good solubility. Understanding how changes in the structure of the ligands can influence the main relaxometric parameters, together with the choice of a good target, enables the rational design of optimal contrast agents. The clinical translation of targeted contrast agents is indeed still scarce, with only few contrast agents reaching clinical trials phase. Moreover, the clinically approved Vasovist® has recently seen its withdrawal from the market due to poor sales.