Structures and Properties of Dinitrosyl Iron and Cobalt Complexes Ligated by Bis(3,5-diisopropyl-1-pyrazolyl)methane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Structure
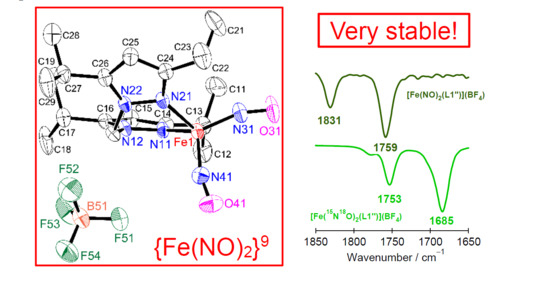
2.3. IR Spectroscopy
2.4. UV–Vis Spectroscopy
2.5. Magnetic Property
2.6. Reactivity Toward Dioxygen
3. Materials and Methods
3.1. Material and General Techniques
3.2. Instrumentation
3.3. Preparation of Complexes
3.3.1. [Fe(NO)2(L1”)](BF4)
3.3.2. [Fe(15N18O)2(L1”)](BF4)
3.3.3. [Co(NO)2(μ-I)]n
3.3.4. [Co(15N18O)2(μ-I)]n
3.3.5. [Co(NO)2(L1”)](BF4)
3.3.6. [Co(15N18O)2(L1”)](BF4)
3.3.7. [FeCl2(L1”)]
3.3.8. [Co(κ2-O2NO)2(L1”)]
3.3.9. [Co(κ2-O2N)2(L1”)]
3.4. O2 Reactivity of the Dinitrosyl Complexes
3.4.1. Reaction of [Fe(NO)2(L1”)](BF4) with O2 in Solution
3.4.2. Reaction of [Fe(NO)2(L1”)](BF4) with O2 in the Solid State
3.4.3. Reaction of [Co(NO)2(L1”)](BF4) with O2 in Solution
3.4.4. Reaction of [Co(NO)2(L1”)](BF4) with O2 in the Solid State
3.5. X-ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ignarro, L. Nitric oxide: Biology and pathobiology; Academic Press: Scan Diego, CA, USA, 2000. [Google Scholar]
- Butler, A.R.; Megson, I.L. Non-heme iron nitrosyls in biology. Chem. Rev. 2002, 102, 1155. [Google Scholar] [CrossRef] [PubMed]
- Wasser, I.M.; de Vries, S.; Moënne-Loccoz, P.; Schröder, I.; Karlin, K.D. Nitric oxide in biological denitrification: Fe/Cu metalloenzyme and metal complex NOx redox chemistry. Chem. Rev. 2002, 102, 1201. [Google Scholar] [CrossRef] [PubMed]
- McCleverty, J.A. Chemistry of nitric oxide relevant to biology. Chem. Rev. 2004, 104, 403. [Google Scholar] [CrossRef] [PubMed]
- Tonzetich, Z.J.; McQuade, L.E.; Lippard, S.J. Detecting and understanding the roles of nitric oxide in biology. Inorg. Chem. 2010, 49, 6338. [Google Scholar] [CrossRef] [PubMed]
- Berto, T.C.; Speelman, A.; Zheng, S.; Lehnert, N. Mono- and dinuclear non-heme iron-nitrosyl complexes: Models for key intermediates in bacterial nitric oxide reductases. Coord. Chem. Rev. 2013, 253, 244. [Google Scholar] [CrossRef]
- Li, L.; Li, L. Recent advances in multinuclear metal nitrosyl complexes. Coord. Chem. Rev. 2005, 249, 2408. [Google Scholar] [CrossRef]
- Lewandowska, H.; Kalinowska, M.; Brzóska, K.; Wójciuk, K.; Wójciuka, G.; Kruszewskia, M. Nitrosyl iron complexes–synthesis, structure and biology. Dalton Trans. 2011, 40, 8273. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-L.; Tsou, C.-C.; Liaw, W.-F. Dinitrosyl iron complexes (DNICs): From biomimetic synthesis and spectroscopic characterization toward unveiling the biological and catalytic roles of DNICs. Acc. Chem. Res. 2015, 48, 1184. [Google Scholar] [CrossRef]
- Pulukkody, R.; Darensbourg, M.Y. Synthetic advances inspired by the bioactive dinitrosyl iron unit. Acc. Chem. Res. 2015, 48, 2049. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Kim, E. Synthetic modeling chemistry of iron-sulfur clusters in nitric oxide signaling. Acc. Chem. Res. 2015, 48, 2453. [Google Scholar] [CrossRef]
- Bello, M.L.; Nuccetelli, M.; Caccuri, A.M.; Stella, L.; Parker, M.W.; Rossjohn, J.; McKinstry, W.J.; Mozzi, A.F.; Federici, G.; Polizio, F.; et al. Human glutathione transferase P1-1 and nitric oxide carriers. J. Biol. Chem. 2001, 276, 42138. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, E.; Parker, L.J.; Pedersen, J.Z.; Nuccetelli, M.; Mazzetti, A.P.; Pastore, A.; Federici, G.; Caccuri, A.M.; Ricci, G.; Adams, J.J.; et al. Nitrosylation of human glutathione transferase P1-1 with dinitrosyl diglutathionyl iron complex in vitro and in vivo. J. Biol. Chem. 2005, 280, 42172. [Google Scholar] [CrossRef] [PubMed]
- Hino, T.; Matsumoto, Y.; Nagano, S.; Sugimoto, H.; Fukumori, Y.; Murata, T.; Iwata, S.; Shiro, Y. Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science 2010, 330, 1666. [Google Scholar] [CrossRef] [PubMed]
- Tosha, T.; Nomura, T.; Nishida, T.; Saeki, N.; Okubayashi, K.; Yamagiwa, R.; Sugahara, M.; Nakane, T.; Yamashita, K.; Hirata, K.; et al. Capturing an initial intermediate during the P450nor enzymatic reaction using time-resolved XFEL crystallography and caged-substrate. Nature Commun. 2017, 8, 1585. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.-Y.; Chung, C.-W.; Santos, J.-H.; Villaflores, O.-B.; Lu, T.-T. Fe in biosynthesis, translocation, and signal transduction of NO: toward bioinorganic engineering of dinitrosyl iron complexes into NO-delivery scaffolds for tissue engineering. Dalton Trans. 2019, 48, 9431. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W.; Schwederski, B. Non-innocent ligands in bioinorganic chemistry–An overview. Coord. Chem. Rev. 1910, 254, 1580. [Google Scholar] [CrossRef]
- Lyaskovskyy, V.; de Bruin, B. Redox non-innocent ligands: Versatile new tools to control catalytic reactions. ACS Catalysis 2012, 2, 270. [Google Scholar] [CrossRef]
- Enemark, J.H.; Feltham, R.D. Principles of structure, bonding, and reactivity for metal nitrosyl complexes. Coord. Chem. Rev. 1974, 13, 339. [Google Scholar]
- Speelman, A.L.; Zhang, B.; Silakov, A.; Skodje, K.M.; Alp, E.E.; Zhao, J.; Hu, M.Y.; Kim, E.; Krebs, C.; Lehnert, N. Unusual synthetic pathway for an {Fe(NO)2}9 dinitrosyl iron complex (DNIC) and insight into DNIC electronic structure via nuclear resonance vibrational spectroscopy. Inorg. Chem. 2016, 55, 5485. [Google Scholar] [CrossRef]
- Tonzetich, Z.J.; Do, L.H.; Lippard, S.J. Dinitrosyl iron complexes relevant to Rieske cluster nitrosylation. J. Am. Chem. Soc. 2009, 131, 7964. [Google Scholar] [CrossRef]
- Ye, S.; Neese, F. The unusual electronic structure of dinitrosyl iron complexes. J. Am. Chem. Soc. 2010, 132, 3646. [Google Scholar] [CrossRef] [PubMed]
- Tonzetich, Z.J.; Héroguel, F.; Do, L.H.; Lippard, S.J. Chemistry of nitrosyliron complexes supported by a β-diketiminate ligand. Inorg. Chem. 2011, 50, 1570. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.L.; Blackwell, H.E.; Brown, N.C.; Connelly, N.G.; Crossley, J.G.; Orpen, A.G.; Rieger, A.L.; Rieger, P.H. Synthesis of the 17-electron cations [FeL(L’)(NO)2]+ (L, L’ = PPh3, OPPh3): Structure and bonding in four-co-ordinate metal dinitrosyls, and implications for the identity of paramagnetic iron dinitrosyl complex catalysts. J. Chem. Soc., Dalton Trans. 1996, 3491. [Google Scholar] [CrossRef]
- Harrop, T.C.; Tonzetich, Z.J.; Reisner, E.; Lippard, S.J. Reactions of synthetic [2Fe-2S] and [4Fe-4S] clusters with nitric oxide and nitrosothiols. J. Am. Chem. Soc. 2008, 130, 15602. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.-H.; Chiou, Y.-M.; Que, L., Jr. Models for extradiol cleaving catechol dioxygenases: syntheses, structures, and reactivities of iron(II)−monoanionic catecholate complexes. Inorg. Chem. 2001, 40, 3181. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.L.; Hsieh, C.-H.; Reibenspies, J.H.; Darensbourg, M.Y. N-Heterocyclic carbene ligands as mimics of imidazoles/histidine for the stabilization of di- and trinitrosyl iron complexes. Inorg. Chem. 2011, 50, 8541. [Google Scholar] [CrossRef] [PubMed]
- Skodje, K.M.; Kwon, M.-Y.; Chung, S.W.; Kim, E. Coordination-triggered NO release from a dinitrosyl iron complex leads to anti-inflammatory activity. Chem. Sci. 2014, 5, 2374. [Google Scholar] [CrossRef]
- Hung, M.-C.; Tsai, M.-C.; Lee, G.-H.; Liaw, W.-F. Transformation and structural discrimination between the neutral {Fe(NO)2}10 dinitrosyliron complexes (DNICs) and the anionic/cationic {Fe(NO)2}9 DNICs. Inorg. Chem. 2006, 45, 6041. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Sundberg, E.B.; Nguyen, P.; Grant, G.P.G.; Sheth, C.; Zhao, Q.; Herron, S.; Kantardjieff, K.A.; Li, L. Synthesis, structures, spectroscopic and electrochemical properties of dinitrosyl iron complexes with bipyridine, terpyridine, and 1,10-phenathroline. Inorg. Chem. 2009, 48, 9779. [Google Scholar] [CrossRef]
- Cho, S.-L.; Liao, C.-J.; Lu, T.-T. Synthetic methodology for preparation of dinitrosyl iron complexes. J. Biol. Inorg. Chem. 2019, 24, 495. [Google Scholar] [CrossRef]
- Fujisawa, K.; Soma, S.; Kurihara, H.; Ohta, A.; Dong, H.T.; Minakawa, Y.; Zhao, J.; Alp, E.E.; Hu, M.Y.; Lehnert, N. Stable ferrous mononitroxyl {FeNO}8 complex with a hindered hydrotris(pyrazolyl)borate coligand: Structure, spectroscopic characterization, and reactivity toward NO and O2. Inorg. Chem. 2019, 58, 4059. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Soma, S.; Kurihara, H.; Dong, H.T.; Bilodeau, M.; Lehnert, N. A cobalt–nitrosyl complex with a hindered hydrotris(pyrazolyl)borate coligand: Detailed electronic structure, and reactivity towards dioxygen. Dalton Trans. 2017, 46, 13273. [Google Scholar] [CrossRef] [PubMed]
- Soma, S.; Stappen, C.V.; Kiss, M.; Szilagyi, R.K.; Lehnert, N.; Fujisawa, K. Distorted tetrahedral nickel-nitrosyl complexes: Spectroscopic characterization and electronic structure. J. Biol. Inorg. Chem. 2016, 21, 757. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Tateda, A.; Miyashita, Y.; Okamoto, K.; Paulat, F.; Praneeth, V.K.K.; Merkle, A.; Lehnert, N. Structural and spectroscopic characterization of mononuclear copper(I) nitrosyl complexes: End-on versus side-on coordination of no to copper(I). J. Am. Chem. Soc. 2008, 130, 1205. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, N.; Cornelissen, U.; Neese, F.; Ono, T.; Noguchi, Y.; Okamoto, K.; Fujisawa, K. Synthesis and spectroscopic characterization of copper(II)-nitrito complexes with hydrotris(pyrazolyl)borate and related coligands. Inorg. Chem. 2007, 46, 3916. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Noguchi, Y.; Miyashita, Y.; Okamoto, K.; Lehnert, N. Mononuclear and binuclear copper(I) complexes ligated by bis(3,5-diisopropyl-1-pyrazolyl)methane: insight into the fundamental coordination chemistry of three-coordinate copper(i) complexes with a neutral coligand. Inorg. Chem. 2007, 46, 10607. [Google Scholar] [CrossRef]
- Fujisawa, K.; Kanda, R.; Miyashita, Y.; Okamoto, K. Copper(II) complexes with neutral bis(pyrazolyl)methane ligands: The influence of steric hindrance on their structures and properties. Polyhedron. 2008, 27, 1432. [Google Scholar] [CrossRef]
- Roustan, J.-L.; Ansari, N.; Page, Y.L.; Charland, J.-P. Molecular geometry of M(NO)2 complexes: single crystal X-ray structure of Co(NO)2(C5H5N)2+BF4−, lability of the pyridine ligands of Co(NO)2(C5H5N)2+, and its relevance to the formation of the Co2(NO)3+ bimetallic core. Can. J. Chem. 1992, 70, 1650. [Google Scholar] [CrossRef]
- Tennyson, A.G.; Dhar, S.; Lippard, S.J. Synthesis and characterization of {Ni(NO)}10 and {Co(NO)2}10 complexes supported by thiolate ligands. J. Am. Chem. Soc. 2008, 130, 15087. [Google Scholar] [CrossRef]
- Crimmin, M.R.; Rosebrugh, L.E.; Tomson, N.C.; Weyhermüller, T.; Bergmana, R.G.; Tostea, L.D.; Wieghardt, K. [(TMEDA)Co(NO)2][BPh4]: A versatile synthetic entry point to four and five coordinate {Co(NO)2}10 complexes. J. Organomet. Chem. 2011, 696, 3981. [Google Scholar] [CrossRef]
- Tomson, N.C.; Crimmin, M.R.; Petrenko, T.; Rosebrugh, L.E.; Sproules, S.; Boyd, W.C.; Bergman, R.G.; DeBeer, S.; Toste, F.D.; Wieghardt, K. A step beyond the Feltham–Enemark notation: Spectroscopic and Correlated ab Initio Computational Support for an Antiferromagnetically Coupled M(II)–(NO)– Description of Tp*M(NO) (M = Co, Ni). J. Am. Chem. Soc. 2011, 133, 18785. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, S.; Incarvito, C.D.; Rheingold, A.L.; Theopold, K.H. In pursuit of a stable peroxynitrite complex–NOx (x = 1–3) derivatives of Tpt-Bu,MeCo. Inorg. Chim. Acta 2003, 345, 333. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Fujisawa, K.; Ibi, N.; Miyashita, Y.; Okamoto, K. Structural and spectroscopic characterization of first-row transition metal(II) substituted blue copper model complexes with hydrotris(pyrazolyl)borate. Inorg. Chem. 2005, 44, 325. [Google Scholar] [CrossRef] [PubMed]
- Mehn, M.P.; Fujisawa, K.; Hegg, E.L.; Que, L., Jr. Oxygen activation by nonheme iron(II) complexes: α-keto carboxylate versus carboxylate. J. Am. Chem. Soc. 2003, 125, 7828. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.G.; Kalyvas, H.; SkodjeK, M.; Hayashi, T.; Moënne-Loccoz, P.; Callan, P.E.; Shearer, J.; Kirschenbaum, L.J.; Kim, E. Phenol nitration induced by an {Fe(NO)2}10 dinitrosyl iron complex. J. Am. Chem. Soc. 2011, 133, 1184. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Dahl, L.F.; de Gil, E.R.; Feltham, R.D. The Solid-state structures of dinitrosyliron iodide and dinitrosylcobalt iodide: the stereochemical consequences of strong metal-metal interactions in ligand-bridged complexes. J. Am. Chem. Soc. 1969, 91, 1653. [Google Scholar] [CrossRef]
- Haymore, B.; Feltham, R.D. Nirosyliron, -cobalt, and -nickel iodides. Inorg. Synth. 1973, 14, 81. [Google Scholar]
- Rigaku Corporation. CrystalClear: Data Collection and Processing Software; Rigaku Corporation: Tokyo, Japan, 2015. [Google Scholar]
- SIR2008: Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: a suite of computer programs for crystal structure solution of proteins. J. Appl. Cryst. 2007, 40, 609. [Google Scholar] [CrossRef]
- Rigaku Corporation. Crystal Structure 4.3: Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2018. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112. [Google Scholar] [CrossRef]
- Parsonsa, S.; Flack, H. Precise absolute-structure determination in light-atom crystals. Acta Cryst. 2004, A60, S61. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crys. 2015, C71, 9. [Google Scholar]








| Complex | ν (N–O)/cm–1 | M–N(O)/Å | N–O/Å | M–N–O/° | Reference |
|---|---|---|---|---|---|
| Four-coordinate {Fe(NO)2}9 | |||||
| [Fe(NO)2(L1”)](BF4) | 1831 (CH2Cl2) | 1.692(4) | 1.168(6) | 160.4(5) | this work |
| 1759 (CH2Cl2) | 1.699(5) | 1.156(6) | 165.1(4) | ||
| [Fe(NO)2(dmp)](OTf) a | 1840 (KBr) | 1.674(6) | 1.177(7) | 170.7(6) | [20] |
| 1746 (KBr) | 1.675(6) | 1.174(7) | 168.3(6) | ||
| [Fe(NO)2(Ar-nacnac)] a | 1761 (C6D6) | 1.6984(18) | 1.177(2) | 162.7(2) | [21,22,23] |
| 1709 (C6D6) | 1.6882(18) | 1.174(2) | 170.1(2) | ||
| [Fe(NO)2(PPh3)2](PF6) | 1814 (CH2Cl2) | 1.661(4) b | 1.160(6) b | 166.2(4) b | [24] |
| 1766 (CH2Cl2) | |||||
| (Et4N)[Fe(NO)2(S-p-tolyl)2] a | 1732 (KBr) | 1.7210(15) | 1.1992(19) | 166.04(14) | [25] |
| 1691 (KBr) | 1.7096(15) | 1.1958(19) | 170.05(14) | ||
| [Fe(NO)2(6-Me3-TPA)](ClO4) a | 1801 (KBr) | 1.699(3) | 1.168(4) | 159.7(4) | [26] |
| 1726 (KBr) | 1.690(3) | 1.165(4) | 162.4(4) | ||
| [Fe(NO)2(NHC-iPr)](BF4) a | 1789 (THF) | na d | na d | na d | [27] |
| 1733 (THF) | na d | na d | na d | ||
| [Fe(NO)2(tmeda)](PF6) a | 1835 (KBr) | na d | na d | na d | [28] |
| 1769 (KBr) | na d | na d | na d | ||
| Four-coordinate {Fe(NO)2}10 | |||||
| [Fe(NO)2(dmp)] a | 1692 (KBr) | na d | na d | na d | [20] |
| 1628 (KBr) | na d | na d | na d | ||
| (PPN)[Fe(NO)2(Ar-nacnac)] a | 1637 (C6D6) | 1.668(5) | 1.191(6) | 163.2(5) | [21] |
| 1580 (C6D6) | 1.649(4) | 1.218(6) | 165.1(5) | ||
| [Fe(NO)2(PPh3)2] | 1714 (CH2Cl2) | 1.650(7) b | 1.190(10) b | 178.2(7) b | [24] |
| 1668 (CH2Cl2) | |||||
| [Fe(NO)2(NHC-iPr)] a | 1664 (THF) | 1.642(3) b | 1.204(3) b | 173.8(2) b | [27] |
| 1679 (THF) | |||||
| [Fe(NO)2(sparteine)] | 1687(THF) | 1.6501(19) | 1.206(6) | 160.1(3) | [29] |
| 1633 (THF) | 1.6430(19) | 1.214(5) | 176.0(3) | ||
| [Fe(NO)2(tmeda)] a | 1698 (THF) | 1.639(3) | 1.188(4) | 169.9(3) | [29] |
| 1644 (THF) | 1.637(3) | 1.197(4) | 166.9(3) | ||
| [Fe(NO)(bipy)] a | 1684 (solid ATR) c | 1.652(4) | 1.183(5) | 169.0(4) | [30] |
| 1619 (solid ATR) c | 1.647(4) | 1.188(5) | 166.7(4) | ||
| Four-coordinate {Fe(NO)2}8 | |||||
| [Fe(NO)(L3)] | 1696 (KBr) | 1.6753(13) | 1.1865(17) | 176.76(18) | [32] |
| Complex | ν (N–O)/cm−1 | M–N(O)/Å | N–O/Å | M–N–O/° | Reference |
|---|---|---|---|---|---|
| Four-coordinate {Co(NO)2}10 | |||||
| [Co(NO)2(L1”)](BF4) | 1875 (CH2Cl2) | 1.654(5) | 1.151(7) | 175.8(7) | This work |
| 1.673(6) | 1.137(10) | 164.6(7) | |||
| 1798 (CH2Cl2) | 1.673(6) | 1.137(10) | 164.6(7) | ||
| 1.673(6) | 1.137(10) | 164.6(7) | |||
| [Co(NO)2(py)2](BF4) | 1876 (CH2Cl2) | 1.654(6) | 1.130(8) | 170.2(6) | [39] |
| 1798 (CH2Cl2) | 1.644(6) | 1.156(8) | 170.1(6) | ||
| (Et4N)[Co(NO)2(SPh)2] a | 1769 (THF) | 1.684(3) | 1.106(3) | 161.4(3) | [40] |
| 1699 (THF) | 1.651(3) | 1.126(3) | 174.9(2) | ||
| [Co(NO)2(Ar-nacnac)] a | 1801 (C6D6) | 1.633(3) | 1.165(5) | 173.0(4) | [23] |
| 1706 (C6D6) | 1.695(4) | 1.166(5) | 150.1(4) | ||
| [Co(NO)2(tmeda)](BPh4) a | 1866 (KBr) | 1.6630(12) | 1.1475(16) | 168.53(13) | [33,41] |
| 1789 (KBr) | 1.6636(12) | 1.1582(15) | 165.83(11) | ||
| Four-coordinate {CoNO}9 | |||||
| [Co(NO)(L3)] | 1732 (KBr) | 1.700(2) | 1.112(3) | 176.1(3) | [33] |
| [Co(NO)(L0)] a,b | 1732 (KBr) | 1.625(5) | 1.161(6) | 173.5(6) | [42] |
| 1.628(5) | 1.167(6) | 175.5(6) | |||
| [Co(NO)(L2)] a | 1732 (KBr) | 1.671(7) | 1.071(9) | 180.000(3) | [43] |
| Complexes | [Fe(NO)2(L1”)](BF4) | [Co(NO)2(L1”)](BF4)∙thf |
|---|---|---|
| CCDC deposition number | 1944313 | 1944314 |
| Empirical Formula | C19H32BF4FeN6O2 | C23H40BF4CoN6O3 |
| Formula Weight | 519.15 | 539.54 |
| Crystal System | monoclinic | monoclinic |
| Space Group | Cc (#9) | Cc (#9) |
| a/Å | 13.093(3) | 11.777(3) |
| b/Å | 19.318(5) | 15.679(3) |
| c/Å | 10.174(3) | 17.388(4) |
| β/° | 90.349(7) | 108.515(4) |
| V/Å3 | 2573.3(12) | 3044.5(12) |
| Z | 4 | 4 |
| Dcalc/g·cm−3 | 1.340 | 1.297 |
| μ(Mo Kα)/cm−1 | 6.404 | 6.213 |
| Temperature/°C | −95 | −95 |
| 2θ range, ° | 6–55 | 6–55 |
| Reflections collected | 21004 | 24068 |
| Unique reflections | 4870 | 6765 |
| Rint | 0.0369 | 0.0366 |
| Number of Variables | 298 | 343 |
| Refls./Para ratio | 16.34 | 19.72 |
| Residuals: R1 (I > 2σ (I)) | 0.0473 | 0.0536 |
| Residuals: R (All reflections) | 0.0506 | 0.0663 |
| Residuals: wR2 (All reflections) | 0.1365 | 0.1596 |
| Good. of fit ind. | 1.042 | 0.923 |
| Flack parameters | 0.041(7) | 0.018(5) |
| (Parsons’ quotients) | 1698 | 2482 |
| Max/min peak,/e Å−3 | 1.07/−0.34 | 1.04/−0.40 |
| Complexes | [FeCl2(L1”)]∙(CH3)2CO | [Co(NO3)2(L1”)] | [Co(NO2)2(L1”)]∙thf |
|---|---|---|---|
| CCDC number | 1944315 | 1944316 | 1944317 |
| Empirical Formula | C22H38Cl2FeN4O | C19H32Co6N6O6 | C23H40CoN6O5 |
| Formula Weight | 501.32 | 499.43 | 539.54 |
| Crystal System | Triclinic | Orthorhombic | monoclinic |
| Space Group | P (#2) | Pbca (#61) | P21/c (#14) |
| a/Å | 8.9680(14) | 16.8808(19) | 10.0033(15) |
| b/Å | 9.8986(15) | 17.1117(18) | 17.788(3) |
| c/Å | 15.939(2) | 18.875(2) | 16.691(3) |
| α/° | 99.7610(17) | — | — |
| β/° | 92.444(4) | — | 94.743(4) |
| γ/° | 107.572(4) | — | — |
| V/Å3 | 1322.7(3) | 5452.2(10) | 2959.8(9) |
| Z | 2 | 8 | 4 |
| Dcalc/g·cm−3 | 1.259 | 1.217 | 1.211 |
| μ(Mo Kα)/cm−1 | 7.905 | 6.695 | 6.191 |
| Temperature/°C | −95 | −95 | −95 |
| 2θ range, ° | 6–55 | 6–55 | 6–55 |
| Reflections collected | 43303 | 42232 | 46976 |
| Unique reflections | 6059 | 6251 | 6795 |
| Rint | 0.0160 | 0.0294 | 0.0824 |
| Number of Variables | 271 | 289 | 316 |
| Refls./Para ratio | 22.36 | 21.63 | 21.50 |
| Residuals: R1 (I > 2 σ (I)) | 0.0274 | 0.0447 | 0.0539 |
| Residuals: R (All reflections) | 0.0293 | 0.0579 | 0.0805 |
| Residuals: wR2 (All reflections) | 0.0755 | 0.1449 | 0.1427 |
| Good. of fit ind. | 1.054 | 1.112 | 1.069 |
| Max/min peak,/e Å−3 | 0.44/−0.42 | 0.87/−0.55 | 0.65/−0.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurihara, H.; Ohta, A.; Fujisawa, K. Structures and Properties of Dinitrosyl Iron and Cobalt Complexes Ligated by Bis(3,5-diisopropyl-1-pyrazolyl)methane. Inorganics 2019, 7, 116. https://doi.org/10.3390/inorganics7100116
Kurihara H, Ohta A, Fujisawa K. Structures and Properties of Dinitrosyl Iron and Cobalt Complexes Ligated by Bis(3,5-diisopropyl-1-pyrazolyl)methane. Inorganics. 2019; 7(10):116. https://doi.org/10.3390/inorganics7100116
Chicago/Turabian StyleKurihara, Haruka, Ayuri Ohta, and Kiyoshi Fujisawa. 2019. "Structures and Properties of Dinitrosyl Iron and Cobalt Complexes Ligated by Bis(3,5-diisopropyl-1-pyrazolyl)methane" Inorganics 7, no. 10: 116. https://doi.org/10.3390/inorganics7100116
APA StyleKurihara, H., Ohta, A., & Fujisawa, K. (2019). Structures and Properties of Dinitrosyl Iron and Cobalt Complexes Ligated by Bis(3,5-diisopropyl-1-pyrazolyl)methane. Inorganics, 7(10), 116. https://doi.org/10.3390/inorganics7100116