Synthesis and Characterization of Catecholato Copper(II) Complexes with Sterically Hindered Neutral and Anionic N3 Type Ligands: Tris(3,5-diisopropyl-1-pyrazolyl)methane and Hydrotris(3,5-diisopropyl-1-pyrazolyl)borate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Structure
2.3. IR Spectroscopy
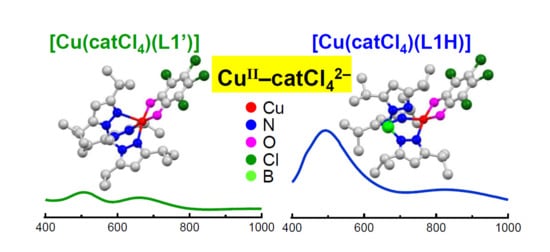
2.4. UV-Vis Spectroscopy
2.5. ESR Spectroscopy
3. Materials and Methods
3.1. Material and General Techniques
3.2. Instrumentation
3.3. Preparation of Complexes
3.3.1. [Cu(catCl4)(L1′)]
3.3.2. [Cu(catBr4)(L1′)]
3.3.3. [Cu(catCl4)(L1H)]
3.4. X-Ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trofimenko, S. Boron-pyrazole chemistry. J. Am. Chem. Soc. 1966, 88, 1842–1844. [Google Scholar] [CrossRef]
- Trofimenko, S.; Calabrese, J.C.; Thompson, J.S. Novel polypyrazolylborate ligands: Coordination control through 3-substituents of the pyrazole ring. Inorg. Chem. 1987, 26, 1507–1514. [Google Scholar] [CrossRef]
- Trofimenko, S. Scorpionates: The Coordination Chemistry of Polypyrazolylborate Ligands; Imperial College Press: London, UK, 1999. [Google Scholar]
- Pettinari, C. Scorpionates II: Chelating Borate Ligands; Imperial College Press: London, UK, 2008. [Google Scholar]
- Trofimenko, S. Geminal poly(1-pyrazolyl)alkanes and their coordination chemistry. J. Am. Chem. Soc. 1970, 92, 5118–5126. [Google Scholar] [CrossRef]
- Juliá, S.; Del Mazo, J.M.; Avila, L.; Elguero, J. Improved synthesis of polyazolylmethanes under solid-liquid phase-transfer catalysis. Org. Prep. Proced. Int. 1984, 16, 299–307. [Google Scholar] [CrossRef]
- Reger, D.L.; Grattan, T.C.; Brown, K.J.; Little, C.A.; Lamba, J.J.S.; Rheingold, A.L.; Sommer, R.D. Syntheses of tris(pyrazolyl)methane ligands and {[tris(pyrazolyl)methane]Mn(CO)3}SO3CF3 complexes: Comparison of ligand donor properties. J. Organomet. Chem. 2000, 607, 120–128. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ono, T.; Aoki, H.; Ishikawa, Y.; Miyashita, Y.; Okamoto, K.; Nakazawa, H.; Higashimura, H. Copper(II) complexes with a novel tris(3,5-diisopropyl-1-pyrazolyl)methane ligand, [Cu(X2){HC(3,5-iPr2pz)3}] (X = Cl and NO3). Inorg. Chem. Commun. 2004, 7, 330–332. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ono, T.; Ishikawa, Y.; Amir, N.; Miyashita, Y.; Okamoto, K.; Lehnert, N. Structural and electronic differences of copper(I) complexes with tris(pyrazolyl)methane and hydrotris(pyrazolyl)borate ligands. Inorg. Chem. 2006, 45, 1698–1713. [Google Scholar] [CrossRef]
- Fujisawa, K.; Iwamoto, H.; Tobita, K.; Miyashita, Y.; Okamoto, K. Copper(II) nitrato and chloro complexes with sterically hindered tridentate ligands: Influence of ligand framework and charge on their structure and physicochemical properties. Inorg. Chim. Acta 2009, 362, 4500–4509. [Google Scholar] [CrossRef]
- Fujisawa, K.; Tobita, K.; Sakuma, S.; Savard, D.; Leznoff, D.B. Binuclear and mononuclear copper(II) chlorido complexes with hindered neutral N3 type ligands: Influence of ligand framework and charge on their structure and physicochemical properties. Inorg. Chim. Acta 2019, 486, 582–588. [Google Scholar] [CrossRef]
- Lehnert, N.; Cornelissen, U.; Neese, F.; Ono, T.; Noguchi, Y.; Okamoto, K.; Fujisawa, K. Synthesis and spectroscopic characterization of copper(II)–nitrito complexes with hydrotris(pyrazolyl)borate and related coligands. Inorg. Chem. 2007, 46, 3916–3933. [Google Scholar] [CrossRef]
- Quist, D.A.; Diaz, D.E.; Liu, J.L.; Karlin, K.D. Activation of dioxygen by copper metalloproteins and insights from model complexes. J. Biol. Inorg. Chem. 2017, 22, 253–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qayyum, M.F.; Sarangi, R.; Fujisawa, K.; Stack, T.D.P.; Karlin, K.D.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. L-Edge X-ray absorption spectroscopy and DFT calculations on Cu2O2 species: Direct electrophilic aromatic attack by side-on peroxo bridged dicopper(II) complexes. J. Am. Chem. Soc. 2013, 135, 17417–17431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirica, L.M.; Vance, M.; Rudd, D.J.; Hedman, B.; Hodgson, K.O.; Solomon, E.I.; Stack, T.D.P. Tyrosinase reactivity in a model complex: An alternative hydroxylation mechanism. Science 2005, 308, 1890–1892. [Google Scholar] [CrossRef] [Green Version]
- Tezgerevska, T.; Alley, K.G.; Boskovic, C. Valence tautomerism in metal complexes: Stimulated and reversible intramolecular electron transfer between metal centers and organic ligands. Coord. Chem. Rev. 2014, 268, 23–40. [Google Scholar] [CrossRef]
- Luca, O.R.; Crabtree, R.H. Redox-active ligands in catalysis. Chem. Soc. Rev. 2013, 42, 1440–1459. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B. Non-innocent ligands in bioinorganic chemistry—An overview. Coord. Chem. Rev. 2010, 254, 1580–1588. [Google Scholar] [CrossRef]
- Ray, K.; Petrenko, T.; Wieghardt, K.; Neese, F. Joint spectroscopic and theoretical investigations of transition metal complexes involving non-innocent ligands. Dalton Trans. 2007, 1552–1566. [Google Scholar] [CrossRef]
- Zanello, P.; Corsini, M. Homoleptic, mononuclear transition metal complexes of 1,2-dioxolenes: Updating their electrochemical-to-structural (X-ray) properties. Coord. Chem. Rev. 2006, 250, 2000–2022. [Google Scholar] [CrossRef]
- Ward, M.D.; McCleverty, J.A. Non-innocent behaviour in mononuclear and polynuclear complexes: Consequences for redox and electronic spectroscopic properties. Dalton Trans. 2002, 275–288. [Google Scholar] [CrossRef]
- Pierpont, C.G. Unique properties of transition metal quinone complexes of the MQ3 series. Coord. Chem. Rev. 2001, 219–221, 415–433. [Google Scholar] [CrossRef]
- Pierpont, C.G. Studies on charge distribution and valence tautomerism in transition metal complexes of catecholate and semiquinonate ligands. Coord. Chem. Rev. 2001, 216–217, 99–125. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.A.; Hao, J.; Rheingold, A.L.; Miller, J.S. High spin ground state copper(II) and nickel(II) complexes possessing the 3,5-di-tert-butyl-1,2-semiquinonate radical anion. Polyhedron 2017, 133, 348–357. [Google Scholar] [CrossRef]
- Verma, P.; Weir, J.; Mirica, L.; Stack, T.D.P. Tale of a twist: Magnetic and optical switching in copper(II) semiquinone complexes. Inorg. Chem. 2011, 50, 9816–9825. [Google Scholar] [CrossRef]
- Rall, J.; Wanner, M.; Albrecht, M.; Hornung, F.; Kaim, W. Sensitive valence tautomer equilibrium of paramagnetic complexes [(L)Cun+(Qn–)] (n = 1 or 2; Q = quinones) related to amine oxidase enzymes. Chem. Eur. J. 1999, 5, 2802–2809. [Google Scholar] [CrossRef]
- Speier, G.; Tisza, S.; Tyeklár, Z.; Lange, C.W.; Pierpont, C.G. Coligand-dependent shifts in charge distribution for copper complexes containing 3,5-di-tert-butylcatecholate and 3,5-di-tert-butylsemiquinonate ligands. Inorg. Chem. 1994, 33, 2041–2045. [Google Scholar] [CrossRef]
- Buchanan, R.M.; Wilson-Blumenberg, C.; Trapp, C.; Larsen, S.K.; Greene, D.L.; Pierpont, C.G. Counterligand dependence of charge distribution in copper-quinone complexes. Structural and magnetic properties of (3,5-di-tert-butylcatecholato)(bipyridine)copper(II). Inorg. Chem. 1986, 25, 3070–3076. [Google Scholar] [CrossRef]
- Ackermann, J.; Meyer, F.; Kaifer, E.; Pritzkow, H. Tuning the activity of catechol oxidase model complexes by geometric changes of the dicopper core. Chem. Eur. J. 2002, 8, 247–258. [Google Scholar] [CrossRef]
- Börzel, H.; Comba, P.; Pritzkow, H. Structural studies on dicopper(II) compounds with catechol oxidase activity. Chem. Commun. 2001, 97–98. [Google Scholar] [CrossRef]
- Berreau, L.M.; Mahapatra, S.; Halfen, J.A.; Houser, R.P.; Young, V.G., Jr.; Tolman, W.B. Reactivity of peroxo- and bis(μ-oxo)dicopper complexes with catechols. Angew. Chem. Int. Ed. 1999, 38, 207–210. [Google Scholar] [CrossRef]
- Bencini, A.; Dei, A.; Sangregorio, C.; Totti, F.; Vaz, M.G.F. Crystal and molecular structure and magnetic exchange properties of bis(di-μ-ethoxo-bis(3,5-di-tert-butylsemiquinonato)dicopper(II)) complex. A synergy between DFT and experimental magnetochemistry. Inorg. Chem. 2003, 42, 8065–8071. [Google Scholar] [CrossRef] [PubMed]
- Ruf, M.; Noll, B.C.; Groner, M.D.; Yee, G.T.; Pierpont, C.G. Pocket semiquinonate complexes of cobalt(II), copper(II), and zinc(II) prepared with the hydrotris(cumenylmethylpyrazolyl)borate ligand. Inorg. Chem. 1997, 36, 4860–4865. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.S.; Calabrese, J.C. Copper-catechol chemistry. Synthesis, spectroscopy, and structure of bis(3,5-di-tert-butyl-o-semiquinato)copper(II). J. Am. Chem. Soc. 1986, 108, 1903–1907. [Google Scholar] [CrossRef]
- Thompson, J.S.; Calabrese, J.C. Synthesis, spectroscopy, and structures of copper(II)-3,5-di-tert-butyl-o-semiquinone complexes. Inorg. Chem. 1985, 24, 3167–3171. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2’-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Rheingold, A.L.; Haggerty, B.S.; Trofimenko, S. The first structurally characterized metal complex involving the free acid of a new tris(pyrazoly1)borate as ligand. Angew. Chem. Int. Ed. Engl. 1994, 33, 1983–1985. [Google Scholar] [CrossRef]
- Kime-Hunt, E.; Spartalian, K.; DeRusha, M.; Nunn, C.M.; Carrano, C.J. Synthesis, characterization, and molecular structures of a series of [(3,5-dimethylpyrazolyl)borato]vanadium(III) and -(IV) complexes. Inorg. Chem. 1989, 28, 4392–4399. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, R.; Hikichi, S.; Akita, M.; Moro-oka, Y. Characterization of a dinuclear Mn(II) tri(μ-carboxylato) complex with the hindered hydrotris(3,5-diisopropyl-1-pyrazolyl)borate (=TpiPr2) ligand: Intramolecular hydrogen bonding interaction between the protonated TpiPr2 and Mn-coordinating carboxylate ligands. Inorg. Chim. Acta 2000, 310, 273–278. [Google Scholar]
- Reinartz, S.; White, P.S.; Brookhart, M.; Templeton, J.L. Structural characterization of an intermediate in arene C–H bond activation and measurement of the barrier to C–H oxidative addition: A platinum(II) η2-benzene adduct. J. Am. Chem. Soc. 2001, 123, 12724–12725. [Google Scholar] [CrossRef]
- Norris, C.M.; Reinartz, S.; White, P.S.; Templeton, J.L. Barriers for arene C–H bond activation in platinum(II) η2-arene intermediates. Organometallics 2002, 21, 5649–5656. [Google Scholar] [CrossRef]
- Kostelansky, C.N.; MacDonald, M.G.; White, P.S.; Templeton, J.L. Stoichiometric alkane dehydrogenation with Tp’PtMe2H to form Tp’Pt(η2-olefin)(H) complexes. Organometallics 2006, 25, 2993–2998. [Google Scholar] [CrossRef]
- Reinartz, S.; White, P.S.; Brookhart, M.; Templeton, J.L. Five-coordinate trispyrazolylborate dihydridosilyl platinum(IV) complexes. J. Am. Chem. Soc. 2001, 123, 6425–6426. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.; Geis, L.; Pintos, V.; Chiozzone, R.; Sanchíz, J.; Hummert, M.; Schumann, H.; Kremer, C. Synthesis, molecular structure and magnetic properties of a rhenium(IV) compound with catechol. J. Mol. Struct. 2009, 921, 80–84. [Google Scholar] [CrossRef]
- Horsman, G.P.; Jirasek, A.; Vaillancourt, F.H.; Barbosa, C.J.; Jarzecki, A.A.; Xu, C.; Mekmouche, Y.; Spiro, T.G.; Lipscomb, J.D.; Blades, M.W.; et al. Spectroscopic studies of the anaerobic enzyme-substrate complex of catechol 1,2-dioxygenase. J. Am. Chem. Soc. 2005, 127, 16882–16891. [Google Scholar] [CrossRef] [Green Version]
- Öhrström, L.; Michaud-Soret, I. Fe–catecholate and Fe–oxalate vibrations and isotopic substitution shifts from DFT quantum chemistry. J. Phys. Chem. A 1999, 103, 256–264. [Google Scholar] [CrossRef]
- Edwards, C.F.; Griffith, W.P.; White, A.J.P.; Williams, D.J. New catecholato oxorhenium(V) complexes. J. Chem. Soc. Dalton Trans. 1992, 957–962. [Google Scholar] [CrossRef]
- Michaud-Soret, I.; Andersson, K.K.; Que, L., Jr. Resonance Raman studies of catecholate and phenolate complexes of recombinant human tyrosine hydroxylase. Biochemistry 1995, 34, 5504–5510. [Google Scholar] [CrossRef]
- Solomon, E.I.; Szilagyi, R.K.; George, S.D.; Basumallick, L. Electronic structures of metal sites in proteins and models: Contributions to function in blue copper proteins. Chem. Rev. 2004, 104, 419–458. [Google Scholar] [CrossRef]
- Hathaway, B.J. A new look at the stereochemistry and electronic properties of complexes of the copper(II) ion. Struct. Bond. 1984, 57, 55–118. [Google Scholar]
- Hathaway, B.J.; Billing, D.E. The electronic properties and stereochemistry of mono-nuclear complexes of the copper(II) ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Kitajima, N.; Fujisawa, K.; Fujimoto, C.; Moro-oka, Y.; Hashimoto, S.; Kitagawa, T.; Toriumi, T.; Tatsumi, K.; Nakamura, A. A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the μ-η2:η2 peroxo dinuclear copper(II) complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = i-Pr and Ph). J. Am. Chem. Soc. 1992, 114, 1277–1291. [Google Scholar] [CrossRef]
- CrystalClear: Data Collection and Processing Software; Rigaku Corporation: Tokyo, Japan, 2015.
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Cryst. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Beurskens, P.T.; Beurskens, G.; de Gelder, R.; García-Granda, S.; Israel, R.; Gould, R.O.; Smits, J.M.M. The DIRDIF99 Program System, Technical Report of the Crystallography Laboratory; University of Nijmegen: Nijmegen, The Netherlands, 1999. [Google Scholar]
- Crystal Structure 4.3: Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2018.
- Sheldrick, G.M. SHELXL Version 2014/3: A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, 71, 9–18. [Google Scholar]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1-yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Water-soluble C-scorpionate complexes—Catalytic and biological applications. Eur. J. Inorg. Chem. 2016, 15–16, 2236–2252. [Google Scholar] [CrossRef]










| Complex | d (Cu–O) / Å | d (C–O) / Å | d (C1–C2) / Å | Reference |
|---|---|---|---|---|
| catecholato complex | ||||
| [Cu(catCl4)(L1′)] | 1.9280(14) 1.9106(18) | 1.316(3) 1.318(2) | 1.425(3) | this work |
| [Cu(catBr4)(L1′)] | 1.929(2) 1.905(3) | 1.318(5) 1.318(4) | 1.421(5) | this work |
| [Cu(catCl4)(L1H)] | 1.890(4) 1.952(4) | 1.336(8) 1.334(6) | 1.412(8) | this work |
| [Cu(catBu2)(bipy)(MeOH)] a | 1.929(2) 1.898(2) | 1.344(4) 1.342(4) | 1.422(4) | [26] |
| [Cu(catBu2)(DBED)] a | 1.943(2) 1.924(2) | 1.347(3) 1.349(3) | 1.425(4) | [27] |
| [Cu(catBu2)(Me3-tacn)] a,b | 1.911(3) 1.903(3) | 1.343(5) 1.346(4) | 1.421(5) | [28] |
| 1.932(2) 1.895(3) | 1.341(5) 1.353(4) | 1.420(5) | ||
| [Cu(catBu2)(py)2](BF4) b | 1.964(2) 1.919(2) | 1.354(4) 1.335(4) | 1.423(6) | [29] |
| 1.964(2) 1.908(2) | 1.367(3) 1.336(4) | 1.408(4) | ||
| [Cu(catBu2)(bpy)] | 1.901(5) 1.870(5) | 1.364(8) 1.338(8) | 1.407(10) | [30] |
| [Cu(catCl4)Cu(H2O)2(μ-py1)](ClO4)2 a | 1.933(2) 1.934(2) | 1.339(4) 1.344(4) | 1.412(5) | [31] |
| [Cu(catCl4)Cu(H2O)(μ-py2)](ClO4)2 a,b | 1.918(3) 1.979(3) | 1.322(6) 1.337(6) | 1.401(7) | [31] |
| 1.939(3) 1.984(3) | 1.336(6) 1.333(5) | 1.407(7) | ||
| [Cu(catCl4)Cu(H2O)(μ-py3)](ClO4)2 a | 1.9684(17).1.9465(17) | 1.333(3) 1.328(3) | 1.421(3) | [31] |
| [Cu(catCl4)(bispidine1)] a | 2.456(2) 1.909(2) | 1.301(3) 1.313(3) | 1.440(3) | [32] |
| [{Cu(catCl4)}2(μ-bispidine2)] a,b | 1.947(4) 1.899(4) | 1.340(7) 1.336(7) | 1.416(9) | [32] |
| 1.914(3) 1.930(5) | 1.316(8) 1.324(6) | 1.429(9) | ||
| [Cu(catCl4)(Bn3-tacn)] a | 1.940(4) 1.915(4) | 1.335(7) 1.317(6) | 1.430(7) | [33] |
| o-semiquinonato complex | ||||
| [Cu(sqBu2)(DPyA)(thf)2](BF4) a | 1.977(3) 1.978(2) | 1.297(4) 1.276(5) | 1.461(5) | [26] |
| [Cu(sqBu2)(bipy)](BF4) a | 1.933(3) 1.936(3) | 1.293(6) 1.287(5) | 1.452(7) | [26] |
| [Cu(sqBu2)(DBED)](SbF6) a | 1.975(2) 1.924(2) | 1.285(3) 1.269(3) | 1.464(4) | [27] |
| [Cu(sqBu2)(TMCD)](SbF6) a | 1.963(2) 1.949(2) | 1.289(3) 1.291(3) | 1.455(4) | [27] |
| [Cu(sqCl4)(Bn3-tacn)] a | 1.988(5) 2.001(4) | 1.272(6) 1.265(7) | 1.435(8) | [33] |
| [Cu(sqBu2)(EtO)]2 b | 1.952(4) 1.949(5) | 1.261(7) 1.294(7) | 1.477(9) | [34] |
| 1.934(5) 1.945(5) | 1.309(8) 1.275(9) | 1.444(9) | ||
| [Cu(sqBu2)(TpCum,Me)] a | 1.952(3) 1.971(3) | 1.279(5) 1.265(6) | 1.457(7) | [35] |
| [Cu(sqBu2)2]2 b | 1.918(4) 1.955(4) | 1.291(7) 1.296(6) | 1.470(8) | [36] |
| 1.944(4) 1.941(4) | 1.290(7) 1.296(6) | 1.45(1) | ||
| [Cu(sqBu2){NH(Py)2}](ClO4)2 a,b | 1.962(5) 1.964(4) | 1.293(7) 1.304(7) | 1.446(9) | [37] |
| 1.934(5) 1.969(4) | 1.289(7) 1.284(4) | 1.45(1) | ||
| Complex | [Cu(catCl4)(L1′)]· 2.5(CH3CN) | [Cu(catBr4)(L1′)]· 2.5(CH3CN) | [Cu(catCl4)(L1H)] |
|---|---|---|---|
| CCDC number | 620467 | 620468 | 620469 |
| Empirical Formula | C39H53.5Cl4CuN8.5O2 | C39H53.5Br4CuN8.5O2 | C33H47BCl4CuN6O2 |
| Formula Weight | 878.77 | 1056.57 | 775.94 |
| Crystal System | Monoclinic | Monoclinic | Monoclinic |
| Space Group | C2/c (#15) | C2/c (#15) | P2/c (#13) |
| a/Å | 19.7412(11) | 19.834(7) | 15.405(7) |
| b/Å | 15.9152(8) | 16.302(5) | 13.137(6) |
| c/Å | 29.637(2) | 29.749(10) | 21.004(10) |
| β/° | 103.9780(9) | 107.918(3) | 101.852(6) |
| V/Å3 | 9035.8(10) | 9152(5) | 4160(3) |
| Z | 8 | 8 | 4 |
| Dcalc/g cm−3 | 1.292 | 1.533 | 1.239 |
| μ(MoKα)/cm−1 | 7.616 | 40.222 | 8.160 |
| Temperature/°C | −71 | −69 | −61 |
| 2θ Range/° | 6–55 | 6–55 | 6–55 |
| Reflections Collected | 30283 | 35994 | 33408 |
| Unique Reflections | 10131 | 10344 | 9475 |
| Rint | 0.0366 | 0.0410 | 0.0710 |
| Number of Variables | 504 | 504 | 424 |
| Reflections/Parameter Ratio | 20.16 | 20.52 | 22.35 |
| Residuals: R1 (I > 2 σ (I)) | 0.0476 | 0.0581 | 0.1099 |
| Residuals: R (All Reflections) | 0.0519 | 0.0768 | 0.1477 |
| Residuals: wR2 (All Reflections) | 0.1187 | 0.1118 | 0.2762 |
| Goodness of Fit Indicator | 1.139 | 1.145 | 1.110 |
| Max/Min Peak/e Å−3 | 0.32/−0.36 | 1.07/−0.68 | 1.05/−0.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, K.; Ono, T.; Okamura, M. Synthesis and Characterization of Catecholato Copper(II) Complexes with Sterically Hindered Neutral and Anionic N3 Type Ligands: Tris(3,5-diisopropyl-1-pyrazolyl)methane and Hydrotris(3,5-diisopropyl-1-pyrazolyl)borate. Inorganics 2020, 8, 37. https://doi.org/10.3390/inorganics8050037
Fujisawa K, Ono T, Okamura M. Synthesis and Characterization of Catecholato Copper(II) Complexes with Sterically Hindered Neutral and Anionic N3 Type Ligands: Tris(3,5-diisopropyl-1-pyrazolyl)methane and Hydrotris(3,5-diisopropyl-1-pyrazolyl)borate. Inorganics. 2020; 8(5):37. https://doi.org/10.3390/inorganics8050037
Chicago/Turabian StyleFujisawa, Kiyoshi, Tetsuya Ono, and Moemi Okamura. 2020. "Synthesis and Characterization of Catecholato Copper(II) Complexes with Sterically Hindered Neutral and Anionic N3 Type Ligands: Tris(3,5-diisopropyl-1-pyrazolyl)methane and Hydrotris(3,5-diisopropyl-1-pyrazolyl)borate" Inorganics 8, no. 5: 37. https://doi.org/10.3390/inorganics8050037
APA StyleFujisawa, K., Ono, T., & Okamura, M. (2020). Synthesis and Characterization of Catecholato Copper(II) Complexes with Sterically Hindered Neutral and Anionic N3 Type Ligands: Tris(3,5-diisopropyl-1-pyrazolyl)methane and Hydrotris(3,5-diisopropyl-1-pyrazolyl)borate. Inorganics, 8(5), 37. https://doi.org/10.3390/inorganics8050037