Recent Progress on Catalysts for the Positive Electrode of Aprotic Lithium-Oxygen Batteries †
Abstract
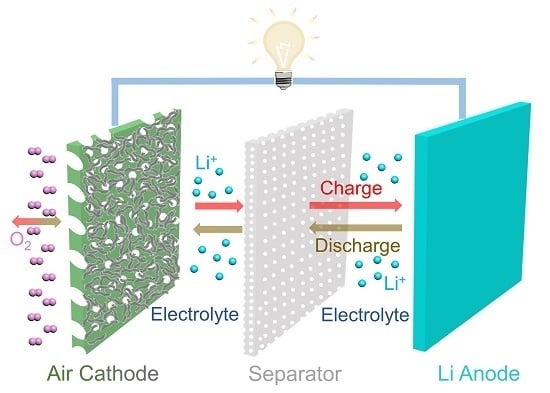
:1. Introduction
2. Overview of Li-O2 Batteries
3. Carbon
3.1. Structural Design
3.2. Heteroatom Doping
3.3. Defect Modification
3.4. Synergy Strategies
4. Noble Metals and Their Oxides
4.1. Adsorption of Discharging Intermediate LiO2
4.2. Stabilization of Discharging Intermediate LiO2
4.3. Utilisation of Bi-Metallic Catalysts
5. Transition Metals and Oxides
5.1. Adsorption of Discharging Intermediate LiO2
5.2. Composite with Carbon
5.3. Lithiation Enhanced Catalytic Performance
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sathiya, M.; Rousse, G.; Ramesha, K.; Laisa, C.P.; Vezin, H.; Sougrati, M.-T.; Doublet, M.L.; Foix, D.; Gonbeau, D.; Walker, W.; et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 2013, 12, 827–835. [Google Scholar] [CrossRef]
- Darwiche, A.; Marino, C.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better cycling performances of bulk Sb in Na-ion batteries compared to Li-ion systems: An unexpected electrochemical mechanism. J. Am. Chem. Soc. 2012, 134, 20805–20811. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Na+/vacancy disordering promises high-rate Na-ion batteries. Acta Phys. Chim. Sin. 2019, 35, 347–348. [Google Scholar]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lu, Y.; Lei, K.; Li, F.; Cheng, F.; Chen, J. Resumption of the discharged Li-AgVO3 primary batteries for rechargeable Li-O2 batteries. Acta Chim. Sin. 2017, 75, 199–205. [Google Scholar] [CrossRef]
- Chen, J. Materials for advanced batteries—A driving force of the mobile information society. Acta Chim. Sin. 2017, 75, 127–128. [Google Scholar] [CrossRef]
- Littauer, E.L.; Tssai, K.C. Anodic behavior of lithium in aqueous electrolytes. J. Electrochem. Soc. 1976, 6, 964–969. [Google Scholar] [CrossRef]
- Abraham, K.M.; Jiang, Z. A polymer electrolyte-based rechargeable lithium/oxygen battery. J. Electrochem. Soc. 1996, 143, 1–5. [Google Scholar] [CrossRef]
- Wang, H.; Xie, K. Investigation of oxygen reduction chemistry in ether and carbonate based electrolytes for Li-O2 batteries. Electrochim. Acta 2012, 64, 29–34. [Google Scholar] [CrossRef]
- Oh, D.; Qi, J.; Lu, Y.-C.; Zhang, Y.; Shao-Horn, Y.; Belcher, A.M. Biologically enhanced cathode design for improved capacity and cycle life for lithium-oxygen batteries. Nat. Commun. 2013, 4, 2756. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Freunberger, S.A.; Chen, Y.; Bruce, P.G. A reversible and higher-rate Li-O2 battery. Science 2012, 337, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yu, T.; Tzoganakis, E.; Amine, K.; Wu, T.; Chen, Z.; Lu, J. Fundamental understanding and material challenges in rechargeable nonaqueous Li-O2 batteries: Recent progress and perspective. Adv. Energy Mater. 2018, 8, 1800348. [Google Scholar] [CrossRef]
- Ottakam Thotiyl, M.M.; Freunberger, S.A.; Peng, Z.; Bruce, P.G. The carbon electrode in nonaqueous Li-O2 cells. J. Am. Chem. Soc. 2013, 135, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Mahne, N.; Schafzahl, B.; Leypold, C.; Leypold, M.; Grumm, S.; Leitgeb, A.; Strohmeier, G.A.; Wilkening, M.; Fontaine, O.; Kramer, D.; et al. Singlet oxygen generation as a major cause for parasitic reactions during cycling of aprotic lithium-oxygen batteries. Nat. Energy 2017, 2, 17036. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Z.; Zou, Q.; Cong, G.; Lu, Y.-C. Mechanistic insights into catalyst-assisted nonaqueous oxygen evolution reaction in lithium-oxygen batteries. J. Phys. Chem. C 2016, 120, 6459–6466. [Google Scholar] [CrossRef]
- Wang, D.; Mu, X.; He, P.; Zhou, H. Materials for advanced Li-O2 batteries: Explorations, challenges and prospects. Mater. Today 2019. [Google Scholar] [CrossRef]
- Shu, C.; Wang, J.; Long, J.; Liu, H.-K.; Dou, S.-X. Understanding the reaction chemistry during charging in aprotic lithium-oxygen batteries: Existing problems and solutions. Adv. Mater. 2019, 1804587. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-X.; Zhu, Q.-C.; Chen, J.-S. Strategies toward high-performance cathode materials for lithium-oxygen batteries. Small 2018, 14, 1800078. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.; Zhang, X. Functional and stability orientation synthesis of materials and structures in aprotic Li-O2 batteries. Chem. Soc. Rev. 2018, 47, 2921–3004. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Zhou, Y.; Dai, W.; Cui, X.; Lai, M.; Wang, L.; Huo, F.; Huang, W.; Hu, Z.; Chen, W. Recent advances in understanding of the mechanism and control of Li2O2 formation in aprotic Li-O2 batteries. Chem. Soc. Rev. 2017, 46, 6046–6072. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Li, C.; Liu, Z.; Chen, Y.; Freunberger, S.A.; Ashok, P.C.; Praveen, B.B.; Dholakia, K.; Tarascon, J.-M.; Bruce, P.G. The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li-O2 batteries. Nat. Chem. 2014, 6, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, Y.; Johnson, L.; Bruce, P.G. Promoting solution phase discharge in Li-O2 batteries containing weakly solvating electrolyte solutions. Nat. Mater. 2016, 15, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Aetukuri, N.B.; McCloskey, B.D.; Garcia, J.M.; Krupp, L.E.; Viswanathan, V.; Luntz, A.C. Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li-O2 batteries. Nat. Chem. 2015, 7, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium-air batteries. Nat. Energy 2016, 1, 16128. [Google Scholar] [CrossRef]
- Peng, Z.; Freunberger, S.A.; Hardwick, L.J.; Chen, Y.; Giordani, V.; Barde, F.; Novak, P.; Graham, D.; Tarascon, J.-M.; Bruce, P.G. Oxygen reactions in a non-aqueous Li+ electrolyte. Angew. Chem. Int. Ed. 2011, 50, 6351–6355. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Ye, S. In situ study of oxygen reduction in dimethyl sulfoxide (DMSO) solution: A fundamental study for development of the lithium-oxygen battery. J. Phys. Chem. C 2015, 119, 12236–12250. [Google Scholar] [CrossRef]
- Frith, J.T.; Russell, A.E.; Garcia-Araez, N.; Owen, J.R. An in-situ Raman study of the oxygen reduction reaction in ionic liquids. Electrochem. Commun. 2014, 46, 33–35. [Google Scholar] [CrossRef]
- Galloway, T.A.; Hardwick, L.J. Utilizing in situ electrochemical SHINERS for oxygen reduction reaction studies in aprotic electrolytes. J. Phys. Chem. Lett. 2016, 7, 2119–2124. [Google Scholar] [CrossRef]
- Zhai, D.; Wang, H.-H.; Lau, K.C.; Gao, J.; Redfern, P.C.; Kang, F.; Li, B.; Indacochea, E.; Das, U.; Sun, H.-H.; et al. Raman evidence for late stage disproportionation in a Li-O2 battery. J. Phys. Chem. Lett. 2014, 5, 2705–2710. [Google Scholar] [CrossRef]
- Olivares-Marin, M.; Sorrentino, A.; Lee, R.-C.; Pereiro, E.; Wu, N.-L.; Tonti, D. Spatial distributions of discharged products of lithium-oxygen batteries revealed by synchrotron X-ray transmission microscopy. Nano Lett. 2015, 15, 6932–6938. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Walter, E.D.; Xu, W.; Nasybulin, E.N.; Bhattacharya, P.; Bowden, M.E.; Engelhard, M.H.; Zhang, J.-G. The mechanisms of oxygen reduction and evolution reactions in nonaqueous lithium-oxygen batteries. ChemSusChem 2014, 7, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, B.; Song, S.; Xu, W.; Zhang, J.-G.; Wang, C. Revealing the reaction mechanisms of Li-O2 batteries using environmental transmission electron microscopy. Nat. Nanotechnol. 2017, 12, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lai, N.-C.; Lu, Y.-R.; Zhou, Y.; Dong, C.-L.; Lu, Y.-C. A solvent-controlled oxidation mechanism of Li2O2 in lithium-oxygen batteries. Joule 2018, 2, 2364–2380. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Shao-Horn, Y. Probing the reaction kinetics of the charge reactions of nonaqueous Li-O2 batteries. J. Phys. Chem. Lett. 2013, 4, 93–99. [Google Scholar] [CrossRef]
- Zhai, D.; Wang, H.-H.; Yang, J.; Lau, K.C.; Li, K.; Amine, K.; Curtiss, L.A. Disproportionation in Li-O2 batteries based on a large surface area carbon cathode. J. Am. Chem. Soc. 2013, 135, 15364–15372. [Google Scholar] [CrossRef]
- Gallant, B.M.; Kwabi, D.G.; Mitchell, R.R.; Zhou, J.; Thompson, C.V.; Shao-Horn, Y. Influence of Li2O2 morphology on oxygen reduction and evolution kinetics in Li-O2 batteries. Energy Environ. Sci. 2013, 6, 2518–2528. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Q.; Zhang, X.; McKee, W.C.; Xu, Y.; Ling, S.; Li, H.; Zhong, G.; Yang, Y.; Peng, Z. Amorphous Li2O2: Chemical synthesis and electrochemical properties. Angew. Chem. Int. Ed. 2016, 55, 10717–10721. [Google Scholar] [CrossRef]
- Tian, F.; Radin, M.D.; Siegel, D.J. Enhanced charge transport in amorphous Li2O2. Chem. Mater. 2014, 26, 2952–2959. [Google Scholar] [CrossRef]
- Rinaldi, A.; Wijaya, O.; Hoster, H.E. Lithium-oxygen cells: An analytical model to explain key features in the discharge voltage profiles. ChemElectroChem 2016, 3, 1944–1950. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Lin, Y.; Moitoso, B.; Martinez-Martinez, C.; Walsh, E.D.; Lacey, S.D.; Kim, J.-W.; Dai, L.; Hu, L.; Connell, J.W. Ultrahigh-capacity lithium-oxygen batteries enabled by dry-pressed holey graphene air cathodes. Nano Lett. 2017, 17, 3252–3260. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yao, Y.; Tang, Z.; Zhao, T.; Wu, F.; Yang, Y.; Huang, Q. Self-nitrogen-doped carbon from plant waste as an oxygen electrode material with exceptional capacity and cycling stability for lithium-oxygen batteries. ACS Appl. Mater. Interfaces 2018, 10, 32212–32219. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; So, J.-Y.; Moon, S.-H.; Han, S.-B.; Choi, S.; Kim, E.-S.; Shin, Y.-K.; Lee, J.-E.; Kwak, D.-H.; Lee, C.; et al. Nature inspired cathodes using high-density carbon papers with an eddy current effect for high-rate performance lithium-air batteries. J. Mater. Chem. A 2018, 6, 9550–9560. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Zhu, J. Binder-free nitrogen-doped carbon paper electrodes derived from polypyrrole/cellulose composite for Li-O2 batteries. J. Power Sources 2016, 306, 559–566. [Google Scholar] [CrossRef]
- Zhu, Q.-C.; Du, F.-H.; Xu, S.-M.; Wang, Z.-K.; Wang, K.-X.; Chen, J.-S. Hydroquinone resin induced carbon nanotubes on Ni foam as binder-free cathode for Li-O2 batteries. ACS Appl. Mater. Interfaces 2016, 8, 3868–3873. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.; Li, C.; Cui, Z.; Guo, X. Job-sharing cathode design for Li-O2 batteries with high energy efficiency enabled by in situ ionic liquid bonding to cover carbon surface defects. J. Mater. Chem. A 2016, 4, 241–249. [Google Scholar] [CrossRef]
- Yu, R.; Fan, W.; Guo, X.; Dong, S. Highly ordered and ultra-long carbon nanotube arrays as air cathodes for high-energy-efficiency Li-oxygen batteries. J. Power Sources 2016, 306, 402–407. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Xu, J.-J.; Chang, Z.-W.; Bao, D.; Yin, Y.-B.; Liu, T.; Yan, J.-M.; Liu, D.-P.; Zhang, Y.; Zhang, X.-B. Blood-capillary-inspired, free-standing, flexible, and low-cost super-hydrophobic N-CNTs@SS cathodes for high-capacity, high-rate, and stable Li-air batteries. Adv. Energy Mater. 2018, 8, 1702242. [Google Scholar] [CrossRef]
- Zhong, X.; Papandrea, B.; Xu, Y.; Lin, Z.; Zhang, H.; Liu, Y.; Huang, Y.; Duan, X. Three-dimensional graphene membrane cathode for high energy density rechargeable lithium-air batteries in ambient conditions. Nano Res. 2017, 10, 472–482. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Liu, S.; Yang, J.; Fan, X.; Huang, H.; Qiu, J. 3D porous N-doped graphene frameworks made of interconnected nanocages for ultrahigh-rate and long-life Li-O2 batteries. Adv. Funct. Mater. 2015, 25, 6913–6920. [Google Scholar] [CrossRef]
- Wu, A.; Shen, S.; Yan, X.; Xia, G.; Zhang, Y.; Zhu, F.; Zhang, J. CxNy particles@N-doped porous graphene: A novel cathode catalyst with a remarkable cyclability for Li-O2 batteries. Nanoscale 2018, 10, 12763–12770. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Guo, X.; Ito, Y.; Liu, P.; Hojo, D.; Aida, T.; Hirata, A.; Fujita, T.; Adschiri, T.; Zhou, H.; et al. Effect of chemical doping on cathodic performance of bicontinuous nanoporous graphene for Li-O2 batteries. Adv. Energy Mater. 2016, 6, 1501870. [Google Scholar] [CrossRef]
- Han, C.-P.; Veeramani, V.; Hsu, C.-C.; Jena, A.; Chang, H.; Yeh, N.-C.; Hu, S.-F.; Liu, R.-S. Vertically-aligned graphene nanowalls grown via plasma-enhanced chemical vapor deposition as a binder-free cathode in Li-O2 batteries. Nanotechnology 2018, 29, 505401. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Wong, R.A.; Park, W.; Yamanaka, K.; Ohta, T.; Jung, Y.; Byon, H.R. Nanostructuring one-dimensional and amorphous lithium peroxide for high round-trip efficiency in lithium-oxygen batteries. Nat. Commun. 2018, 9, 680. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Chen, S.; Liu, H.; Wang, G. Mesoporous carbon nanocube architecture for high-performance lithium-oxygen batteries. Adv. Funct. Mater. 2015, 25, 4436–4444. [Google Scholar] [CrossRef]
- Shu, C.; Lin, Y.; Su, D. N-doped onion-like carbon as an efficient oxygen electrode for long-life Li-O2 battery. J. Mater. Chem. A 2016, 4, 2128–2136. [Google Scholar] [CrossRef]
- Xie, J.; Yao, X.; Cheng, Q.; Madden, I.P.; Dornath, P.; Chang, C.-C.; Fan, W.; Wang, D. Three dimensionally ordered mesoporous carbon as a stable, high-performance Li-O2 battery cathode. Angew. Chem. Int. Ed. 2015, 54, 4299–4303. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, E.; Ahn, W.-S.; Shim, S.E. Controlling porosity of porous carbon cathode for lithium oxygen batteries: Influence of micro and meso porosity. J. Power Sources 2018, 389, 20–27. [Google Scholar] [CrossRef]
- Jung, C.Y.; Zhao, T.S.; An, L.; Zeng, L.; Wei, Z.H. Screen printed cathode for non-aqueous lithium-oxygen batteries. J. Power Sources 2015, 297, 174–180. [Google Scholar] [CrossRef]
- Balaish, M.; Ein-Eli, Y. Meso-pores carbon nano-tubes (CNTs) tissues-perfluorocarbons (PFCs) hybrid air-electrodes for Li-O2 battery. J. Power Sources 2018, 379, 219–227. [Google Scholar] [CrossRef]
- Luo, J.; Yao, X.; Yang, L.; Han, Y.; Chen, L.; Geng, X.; Vattipalli, V.; Dong, Q.; Fan, W.; Wang, D.; et al. Free-standing porous carbon electrodes derived from wood for high-performance Li-O2 battery applications. Nano Res. 2017, 10, 4318–4326. [Google Scholar] [CrossRef]
- Sun, Y. Lithium ion conducting membranes for lithium-air batteries. Nano Energy 2013, 2, 801–816. [Google Scholar] [CrossRef]
- Shao, Y.; Ding, F.; Xiao, J.; Zhang, J.; Xu, W.; Park, S.; Zhang, J.-G.; Wang, Y.; Liu, J. Making Li-air batteries rechargeable: Material challenges. Adv. Funct. Mater. 2013, 23, 987–1004. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, T.; Tan, P.; Wei, Z.; Wu, M. A high-performance solid-state lithium-oxygen battery with a ceramic-carbon nanostructured electrode. Nano Energy 2016, 26, 565–576. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Yao, Y.; Wu, F.; Yu, Y.; Zhang, C. New application of waste citrus maxima peel-derived carbon as an oxygen electrode material for lithium oxygen batteries. ACS Appl. Mater. Interfaces 2018, 10, 32058–32066. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Kim, S.; Kweon, H.; Moon, S.; Lee, C.-H.; Kim, H. Preparation of graphene hollow spheres from vacuum residue of ultra-heavy oil as an effective oxygen electrode for Li-O2 batteries. J. Mater. Chem. A 2018, 6, 4040–4047. [Google Scholar] [CrossRef]
- Lin, X.; Yuan, R.; Cai, S.; Jiang, Y.; Lei, J.; Liu, S.-G.; Wu, Q.-H.; Liao, H.-G.; Zheng, M.; Dong, Q. An open-structured matrix as oxygen cathode with high catalytic activity and large Li2O2 accommodations for lithium-oxygen batteries. Adv. Energy Mater. 2018, 8, 1800089. [Google Scholar] [CrossRef]
- Woo, H.; Kang, J.; Kim, J.; Kim, C.; Nam, S.; Park, B. Development of carbon-based cathodes for Li-air batteries: Present and future. Electron. Mater. Lett. 2016, 12, 551–567. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, Z. Computational insights into oxygen reduction reaction and initial Li2O2 nucleation on pristine and N-doped graphene in Li-O2 batteries. ACS Catal. 2015, 5, 4309–4317. [Google Scholar] [CrossRef]
- Wu, F.; Xing, Y.; Li, L.; Qian, J.; Qu, W.; Wen, J.; Miller, D.; Ye, Y.; Chen, R.; Amine, K.; et al. Facile synthesis of boron-doped rGO as cathode material for high energy Li-O2 batteries. ACS Appl. Mater. Interfaces 2016, 8, 23635–23645. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, W.; Wu, N.; Wang, X.; Zhang, T.; Chen, L.; Zeng, R.; Wang, Y.; Lu, J.; Fu, L.; et al. Tuning the morphology of Li2O2 by noble and 3d metals: A planar model electrode study for Li-O2 battery. ACS Appl. Mater. Interfaces 2017, 9, 19800–19806. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T.; Wang, X.; Chen, L.; Wu, N.; Liu, W.; Lu, H.; Xiao, L.; Fu, L.; Zhuang, L. Tuning the morphology and crystal structure of Li2O2: A graphene model electrode study for Li-O2 battery. ACS Appl. Mater. Interfaces 2016, 8, 21350–21357. [Google Scholar] [CrossRef]
- Cho, S.M.; Yom, J.H.; Hwang, S.W.; Seong, I.W.; Kim, J.; Cho, S.H.; Yoon, W.Y. Morphology control of lithium peroxide using Pd3Co as an additive in aprotic Li-O2 batteries. J. Power Sources 2017, 342, 427–434. [Google Scholar] [CrossRef]
- Cao, C.; Yan, Y.; Zhang, H.; Xie, J.; Zhang, S.; Pan, B.; Cao, G.; Zhao, X. Controlled growth of Li2O2 by cocatalysis of mobile Pd and Co3O4 nanowire arrays for high-performance Li-O2 batteries. ACS Appl. Mater. Interfaces 2016, 8, 31653–31660. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Si, W.; Sun, X.; Liu, B.; Zhang, L.; Yan, C.; Schmidt, O.G. Pd-functionalized MnOx-GeOy nanomembranes as highly efficient cathode materials for Li-O2 batteries. Nano Energy 2016, 19, 428–436. [Google Scholar] [CrossRef]
- Wu, F.; Xing, Y.; Zeng, X.; Yuan, Y.; Zhang, X.; Shahbazian-Yassar, R.; Wen, J.; Miller, D.J.; Li, L.; Chen, R.; et al. Platinum-coated hollow graphene nanocages as cathode used in lithium-oxygen batteries. Adv. Funct. Mater. 2016, 26, 7626–7633. [Google Scholar] [CrossRef]
- Ryu, W.-H.; Gittleson, F.S.; Li, J.; Tong, X.; Taylor, A.D. A new design strategy for observing lithium oxide growth-evolution interactions using geometric catalyst positioning. Nano Lett. 2016, 16, 4799–4806. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Hu, J.; Xie, J.; Cao, G.; Zhang, S.; Yang, S.A.; Zhao, X.; Yang, H.Y. Au-decorated cracked carbon tube arrays as binder-free catalytic cathode enabling guided Li2O2 inner growth for high-performance Li-O2 batteries. Adv. Funct. Mater. 2016, 26, 7725–7732. [Google Scholar] [CrossRef]
- Liu, S.; Wang, G.; Tu, F.; Xie, J.; Yang, H.Y.; Zhang, S.; Zhu, T.; Cao, G.; Zhao, X. Au-nanocrystals-decorated δ-MnO2 as an efficient catalytic cathode for high-performance Li-O2 batteries. Nanoscale 2015, 7, 9589–9596. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, B.; Zhang, J.; Liu, H.; Wang, G. Ruthenium decorated hierarchically ordered macro-mesoporous carbon for lithium oxygen batteries. J. Mater. Chem. A 2016, 4, 9774–9780. [Google Scholar] [CrossRef]
- Lu, X.; Hao, G.-P.; Sun, X.; Kaskel, S.; Schmidt, O.G. Highly dispersed metal and oxide nanoparticles on ultra-polar carbon as efficient cathode materials for Li-O2 batteries. J. Mater. Chem. A 2017, 5, 6284–6291. [Google Scholar] [CrossRef]
- Jung, C.Y.; Zhao, T.S.; Zeng, L.; Tan, P. Vertically aligned carbon nanotube-ruthenium dioxide core–shell cathode for non-aqueous lithium-oxygen batteries. J. Power Sources 2016, 331, 82–90. [Google Scholar] [CrossRef]
- Lu, J.; Lee, Y.J.; Luo, X.; Lau, K.C.; Asadi, M.; Wang, H.-H.; Brombosz, S.; Wen, J.; Zhai, D.; Chen, Z.; et al. A lithium-oxygen battery based on lithium superoxide. Nature 2016, 529, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Liang, X.; Yin, P.; Wang, J.; Lee, Y.L.; Hu, Z.; Liu, X. An amorphous LiO2-based Li-O2 battery with low overpotential and high rate capability. Nano Energy 2017, 41, 535–542. [Google Scholar] [CrossRef]
- Jung, J.; Song, K.; Bae, Y.; Choi, S.-I.; Park, M.; Cho, E.; Kang, K.; Kang, Y.-M. Achieving outstanding Li+-ORR and -OER activities via edge- and corner-embedded bimetallic nanocubes for rechargeable Li-O2 batteries. Nano Energy 2015, 18, 71–80. [Google Scholar] [CrossRef]
- Choi, S.-I.; Xie, S.; Shao, M.; Odell, J.H.; Lu, N.; Peng, H.-C.; Protsailo, L.; Guerrero, S.; Park, J.; Xia, X.; et al. Synthesis and characterization of 9 nm Pt–Ni octahedra with a record high activity of 3.3 A/mg(Pt) for the oxygen reduction reaction. Nano Lett. 2013, 13, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, J.; Sun, K.; Zou, S.; Fang, J. Enhancing by weakening: Electrooxidation of methanol on Pt3Co and Pt nanocubes. Angew. Chem. Int. Ed. 2010, 49, 6848–6851. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Jung, S.C.; Kim, H.-J.; Han, Y.-K.; Oh, S.H. Maximum catalytic activity of Pt3M in Li-O2 batteries: M=Group V transition metals. Nano Energy 2016, 27, 1–7. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jung, S.C.; Han, Y.-K.; Oh, S.H. An atomic-level strategy for the design of a low overpotential catalyst for Li-O2 batteries. Nano Energy 2015, 13, 679–686. [Google Scholar] [CrossRef]
- Luo, X.; Ge, L.; Ma, L.; Kropf, A.J.; Wen, J.; Zuo, X.; Ren, Y.; Wu, T.; Lu, J.; Amine, K. Effect of componential proportion in bimetallic electrocatalysts on the aprotic lithium-oxygen battery performance. Adv. Energy Mater. 2018, 8, 1703230. [Google Scholar] [CrossRef]
- Ma, L.; Luo, X.; Kropf, A.J.; Wen, J.; Wang, X.; Lee, S.; Myers, D.J.; Miller, D.; Wu, T.; Lu, J.; et al. Insight into the catalytic mechanism of bimetallic platinum-copper core–shell nanostructures for nonaqueous oxygen evolution reactions. Nano Lett. 2016, 16, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lyu, Z.; Wang, L.; Dong, W.; Dai, W.; Cui, X.; Hao, Z.; Lai, M.; Chen, W. Co3O4 functionalized porous carbon nanotube oxygen-cathodes to promote Li2O2 surface growth for improved cycling stability of Li-O2 batteries. J. Mater. Chem. A 2017, 5, 25501–25508. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Xu, J.; Xu, D.; Chang, Z.; Yin, Y.; Liu, W.; Zhang, X. Hierarchical Co3O4 porous nanowires as an efficient bifunctional cathode catalyst for long life Li-O2 batteries. Nano Res. 2015, 8, 576–583. [Google Scholar] [CrossRef]
- Lee, S.; Lee, G.-H.; Lee, H.J.; Dar, M.A.; Kim, D.-W. Fe-based hybrid electrocatalysts for nonaqueous lithium-oxygen batteries. Sci. Rep. 2017, 7, 9495. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, Z.; Cheng, F.; Tao, Z.; Chen, J. Micro-nano structured Ni-MOFs as high-performance cathode catalyst for rechargeable Li-O2 batteries. Nanoscale 2015, 7, 11833–11840. [Google Scholar] [CrossRef]
- Shang, C.; Dong, S.; Hu, P.; Guan, J.; Xiao, D.; Chen, X.; Zhang, L.; Gu, L.; Cui, G.; Chen, L. Compatible interface design of CoO-based Li-O2 battery cathodes with long-cycling stability. Sci. Rep. 2015, 5, 8335. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, C.; Cong, L.; Zhang, Y.; Sun, G.; Xie, H.; Sun, L.; Liu, J. Yolk-shell Co2CrO4 nanospheres as highly active catalysts for Li-O2 batteries: Understanding the electrocatalytic mechanism. J. Mater. Chem. A 2017, 5, 544–553. [Google Scholar] [CrossRef]
- Lyu, Z.; Yang, L.; Luan, Y.; Wang, X.R.; Wang, L.; Hu, Z.; Lu, J.; Xiao, S.; Zhang, F.; Wang, X.; et al. Effect of oxygen adsorbability on the control of Li2O2 growth in Li-O2 batteries: Implications for cathode catalyst design. Nano Energy 2017, 36, 68–75. [Google Scholar] [CrossRef]
- Yang, C.; Wong, R.A.; Hong, M.; Yamanaka, K.; Ohta, T.; Byon, H.R. Unexpected Li2O2 film growth on carbon nanotube electrodes with CeO2 nanoparticles in Li-O2 batteries. Nano Lett. 2016, 16, 2969–2974. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Qian, R.; Wen, Z. Atomic-Thick TiO2(B) nanosheets decorated with ultrafine Co3O4 nanocrystals as a highly efficient catalyst for lithium-oxygen battery. ACS Appl. Mater. Interfaces 2018, 10, 41398–41406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, G.; Jin, J.; Zhang, L.; Wen, Z.; Yang, J. Self-catalyzed decomposition of discharge products on the oxygen vacancy sites of MoO3 nanosheets for low-overpotential Li-O2 batteries. Nano Energy 2017, 36, 186–196. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, B.; Bai, Z.; Guo, R.; Xu, Z.-L.; Sadighi, Z.; Qin, L.; Zhang, T.-Y.; Chen, G.; Huang, B.; et al. Anomalous enhancement of Li-O2 battery performance with Li2O2 films assisted by NiFeOx nanofiber catalysts: Insights into morphology control. Adv. Funct. Mater. 2016, 26, 8290–8299. [Google Scholar] [CrossRef]
- Fan, W.; Wang, B.; Guo, X.; Kong, X.; Liu, J. Nanosize stabilized Li-deficient Li2−xO2 through cathode architecture for high performance Li-O2 batteries. Nano Energy 2016, 27, 577–586. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Liu, L.; Liu, Y.; Chou, S.; Shi, D.; Liu, H.; Wu, Y.; Zhang, W.; Chen, J. Mo2C/CNT: An efficient catalyst for rechargeable Li-CO2 batteries. Adv. Funct. Mater. 2017, 27, 1700564. [Google Scholar] [CrossRef]
- Cong, Y.; Geng, Z.; Sun, Y.; Yuan, L.; Wang, X.; Zhang, X.; Wang, L.; Zhang, W.; Huang, K.; Feng, S. Cation segregation of A-site deficiency perovskite La0.85FeO3−δ nanoparticles toward high-performance cathode catalysts for rechargeable Li-O2 battery. ACS Appl. Mater. Interfaces 2018, 10, 25465–25472. [Google Scholar] [CrossRef] [PubMed]
- Sadighi, Z.; Huang, J.; Qin, L.; Yao, S.; Cui, J.; Kim, J.-K. Positive role of oxygen vacancy in electrochemical performance of CoMn2O4 cathodes for Li-O2 batteries. J. Power Sources 2017, 365, 134–147. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Li, W.; Guo, Q.; Gao, H.; You, Y.; Li, Y.; Cui, Z.; Jiang, K.-C.; Long, H.; et al. Nitrogen-doped perovskite as a bifunctional cathode catalyst for rechargeable lithium oxygen batteries. ACS Appl. Mater. Interfaces 2018, 10, 5543–5550. [Google Scholar] [CrossRef]
- Bae, Y.; Ko, D.-H.; Lee, S.; Lim, H.-D.; Kim, Y.-J.; Shim, H.-S.; Park, H.; Ko, Y.; Park, S.K.; Kwon, H.J.; et al. Enhanced stability of coated carbon electrode for Li-O2 batteries and its limitations. Adv. Energy Mater. 2018, 8, 1702661. [Google Scholar] [CrossRef]
- Gong, H.; Xue, H.; Wang, T.; Guo, H.; Fan, X.; Song, L.; Xia, W.; He, J. High-loading nickel cobaltate nanoparticles anchored on three-dimensional N-doped graphene as an efficient bifunctional catalyst for lithium-oxygen batteries. ACS Appl. Mater. Interfaces 2016, 8, 18060–18068. [Google Scholar] [CrossRef]
- Hyun, S.; Shanmugam, S. Mesoporous Co-CoO/N-CNR nanostructures as high-performance air cathode for lithium-oxygen batteries. J. Power Sources 2017, 354, 48–56. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, W.; Wu, S.; Yang, X.; Xia, W.; Shen, Y.; Huang, Y.; Cao, A.; Yuan, Q. Perovskite-type LaSrMnO electrocatalyst with uniform porous structure for an efficient Li-O2 battery cathode. ACS Nano 2016, 10, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, J.; Li, T.; Wang, J.; Gregory, D.H.; Chen, J. MCNTs@MnO2 nanocomposite Cathode Integrated with Soluble O2-Carrier Co-salen in Electrolyte for High-Performance Li-air batteries. Nano Lett. 2017, 17, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-W.; Choi, D.-W.; Lee, C.K.; Yoon, K.R.; Yu, S.; Cheong, J.Y.; Kim, C.; Cho, S.-H.; Park, J.-S.; Park, Y.J.; et al. Rational design of protective In2O3 layer-coated carbon nanopaper membrane: Toward stable cathode for long-cycle Li-O2 batteries. Nano Energy 2018, 46, 193–202. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, L.; Lv, J.; Li, C.; Sun, K. A graphitic foam framework with hierarchical pore structure as self-supported electrodes of Li-O2 batteries and Li ion batteries. J. Mater. Chem. A 2016, 4, 1399–1407. [Google Scholar] [CrossRef]
- Zhu, J.; Metzger, M.; Antonietti, M.; Fellinger, T.-P. Vertically aligned two-dimensional graphene-metal hydroxide hybrid arrays for Li-O2 batteries. ACS Appl. Mater. Interfaces 2016, 8, 26041–26050. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Cui, Z.; Sun, J.; Huo, H.; Chen, C.; Guo, X. Formation of nanosized defective lithium peroxides through Si-coated carbon nanotube cathodes for high energy efficiency Li-O2 batteries. ACS Appl. Mater. Interfaces 2018, 10, 18754–18760. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cheng, F.; Chen, C.; Hu, Y.; Chen, J. Uniform MnO2 nanostructures supported on hierarchically porous carbon as efficient electrocatalysts for rechargeable Li-O2 batteries. Nano Res. 2015, 8, 156–164. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, Z.; Lu, Y.; Wu, X.; Yang, J. Highly active mixed-valent MnOx spheres constructed by nanocrystals as efficient catalysts for long-cycle Li-O2 batteries. J. Mater. Chem. A 2016, 4, 17129–17137. [Google Scholar] [CrossRef]
- Adpakpang, K.; Oh, S.M.; Agyeman, D.A.; Jin, X.; Jarulertwathana, N.; Kim, I.Y.; Sarakonsri, T.; Kang, Y.-M.; Hwang, S.-J. Holey 2D nanosheets of low-valent manganese oxides with an excellent oxygen catalytic activity and a high functionality as a catalyst for Li-O2 batteries. Adv. Funct. Mater. 2018, 28, 1707106. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, T.; Cheng, F.; Zhao, Q.; Han, X.; Chen, J. Recycling application of Li-MnO2 batteries as rechargeable lithium-air batteries. Angew.Chem. Int. Ed. 2015, 54, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Dai, Z.; Lu, J.; Zeng, X.; Yuan, Y.; Bi, X.; Ma, L.; Wu, T.; Yan, Q.; Amine, K. Lithiation-induced non-noble metal nanoparticles for Li-O2 batteries. ACS Appl. Mater. Interfaces 2019, 11, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yan, P.; Xu, W.; Zheng, J.; He, Y.; Luo, L.; Bowden, M.E.; Wang, C.-M.; Zhang, J.-G. Electrochemically formed ultrafine metal oxide nanocatalysts for high-performance lithium-oxygen batteries. Nano Lett. 2016, 16, 4932–4939. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, Z.; Pang, Y.; Sun, Y.; Su, X.; Wang, Y.; Xia, Y. Three-dimensional ordered macroporous FePO4 as high-efficiency catalyst for rechargeable Li-O2 batteries. ACS Appl. Mater. Interfaces 2016, 8, 31638–31645. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ganapathy, S.; Xu, Y.; Zhu, Q.; Chen, W.; Kochetkov, I.; George, C.; Nazar, L.F.; Wagemaker, M. Fe2O3 nanoparticle seed catalysts enhance cyclability on deep (dis)charge in aprotic Li-O2 batteries. Adv. Energy Mater. 2018, 8, 1703513. [Google Scholar] [CrossRef]
- Lee, G.-H.; Sung, M.-C.; Kim, J.-C.; Song, H.J.; Kim, D.-W. Synergistic effect of CuGeO3/graphene composites for efficient oxygen-electrode electrocatalysts in Li-O2 batteries. Adv. Energy Mater. 2018, 8, 1801930. [Google Scholar] [CrossRef]






| Positive Electrode | Cycle Number/Capacity (mAh·g−1)/Rate (mA·g−1) | The First Charge Potential (V) 1 | Maximal Capacity (mAh·g−1) | Maximal Rate (mA·g−1)/Corresponding Charge Potential (V) 2 | Electrolyte | Ref. |
|---|---|---|---|---|---|---|
| Carbon materials | ||||||
| C-800 (nearly 96% microporous) | 120/1000/200 | 3.51 | 6003 | -/- | 1.0 M LiNO3 in DMAc | [59] |
| CMPACs (citrus maxima peel) | 466/500/- | ~4.05 | 7800 | -/- | 1.0 M LiTFSI in TEGDME | [66] |
| N-PIACs (poplar inflorescence) | 220/1000/- | ~3.5 | 12060 | -/- | 1.0 M LiCF3SO3 in TEGDME | [43] |
| Screen-printed cathode | 40/1000/- | 4.06 | 6840 | -/- | 1.0 M LiTFSI in TEGDME | [60] |
| P-CNF/CP | 30/1000/200 | ~4.28 | 5312.51 | 500/~4.38 | 1.0 M LiTFSI in TEGDME | [44] |
| NCPE (N-doped carbon paper) | 30/480/~35 | ~3.47 | 8040 | ~140/~3.7 | 1.0 M LiClO4 in DMSO | [45] |
| CNTs/NF | 110/2000/200 | 3.4 | 12960 | 2000/4.4 | 1.0 M LiTFSI in TEGDME | [46] |
| VACNTs-Ta | >65/1000/200 | ~4.3 | >10000 | 1000/~4.3 | 1.0 M LiTFSI in TEGDME | [48] |
| N-CNTs@SS | 232/500/500 | ~3.75 | 9299 | 2000/- | 0.5 M LiTFSI in PMIMTFSI | [49] |
| Dry-pressed hG | 20/200/20 | ~4.15 | ~4000 | -/- | 1.0 M LiTFSI in TEGDME | [42] |
| 3-D graphene membrane | >2000/140/2800 | ~3.5 | >5700 | -/- | 1.0 M LiNO3 in DMAc | [50] 3 |
| CB-H (graphene hollow spheres) | 104/500/300 | 3.9 | 16805 | 1000/~4.05 | 1.0 M LiNO3 in DMAc | [67] |
| NPGAs (N-doped graphene aerogels) | 72/1000/300 | 4.12 | 10081 | 3200/- | 1.0 M LiTFSI in TEGDME | [51] |
| CxNy@NPG | 200/1000/1000 | 4.1 | 16705 | 1000/~4.25 | 1.0 M LiTFSI in TEGDME | [52] |
| S-doped graphene | 300/1000/300 | ~4.4 | 4920 | 1000/~4.55 | 1.0 M LiTFSI in TEGDME | [53] |
| N-doped graphene | 100/1000/300 | 4.25 | 10400 | -/- | 1.0 M LiTFSI in TEGDME | [53] |
| VA-G/NF | 10/280/140 | 3.4 | - | -/- | 1.0 M LiTFSI in TEGDME | [54] |
| B-rGO | 35/1000/100 | ~3.88 | ~18000 | 2000/~4.15 | 1.0 M LiTFSI in TEGDME | [71] |
| CMK-3 | 19/500/50 | 3.2 | - | 2000/3.8 | 0.5 M LiTFSI in TEGDME | [55] |
| MCCs (meso-porous carbon nanocubes) | 5/1000/400 | 3.75 | 26100 | 2000/- | 0.1 M LiClO4 in DMSO | [56] |
| N-OLC (N-doped onion-like carbon) | 194/1000/1000 | 3.51 | 12180 | 2000/- | 1.0 M LiTFSI in TEGDME | [57] |
| CNPs/CNFs-LATP | 1174/~141.5/~94.3 | ~3.9 | - | -/- | LATP (Li1.3Al0.3-Ti1.7(PO4)3) | [65] |
| Noble metals and their oxides | ||||||
| Pt-HGNs | 10/1000/100 | 3.1 | ~5600 | 500/3.35 | 1.0 M LiTFSI in TEGDME | [77] |
| Pt–Cu (2.5% Pt + 5% Cu) | 35/500/100 | 3.15 | 2376 | -/- | 1.0 M LiCF3SO3 in TEGDME | [91] |
| Pt3Co NCs | 65/1000/200 | 3.1 | 11000 | 2000/- | 1.0 M LiCF3SO3 in TEGDME | [86] |
| MmC@Ru | 100/1000/400 | 3.4 | 12400 | 1000/3.55 | 0.5 M LiClO4 in DMSO | [81] |
| VACNT@RuO2 | 100/~1613/~806.5 | 3.6 | 9050 | ~1613/~4 | 1.0 M LiTFSI in TEGDME | [83] |
| RuOx-CNp@In2O3 | 165/1000/400 | ~4 | ~20000 | 2000/~4.6 | 1.0 M LiTFSI in TEGDME | [114] |
| Pd/UPC | 231/1000/300 | 3.35 | - | 500/3.95 | 1.0 M LiCF3SO3 in TEGDME | [82] |
| RuO2/UPC | 150/1000/300 | 3.6 | - | 500/3.7 | 1.0 M LiCF3SO3 in TEGDME | [82] |
| MGP-20 (MnOx-GeOy-Pd) | 163/1000/300 | 3.1 | 10900 | 500/3.5 | 1.0 M LiCF3SO3 in TEGDME | [76] |
| Pd-rGO | 100/500/1000 | 3.25 | 18687 | 2000/- | 1.0 M LiTFSI in TEGDME | [85] |
| Co3O4 NAs/Pd NCs | 258/500/100 | 3.75 | 5337 | -/- | 1.0 M LiClO4 in DMSO | [75] |
| Ir-rGO | ~40/1000/100 | ~3.62 | ~9500 | -/- | 1.0 M LiCF3SO3 in TEGDME | [84] |
| Au@CST | 112/500/400 | 3.8 | 5488 | 1000/~4.4 | 1.0 M LiClO4 in DMSO | [79] |
| Au-δMnO2 | 165/500/400 | 3.8 | 10600 | 1600/3.9 | 1.0 M LiClO4 in TEGDME | [80] |
| Transition metals and their oxides | ||||||
| Mixed-valent MnOx spheres | 320/1000/100 | 3.5 | 9709 | 800/3.6 | 1.0 M LiCF3SO3 in TEGDME | [119] |
| Li0.50MnO2 | 197/1000/200 | 3.6 | 10823 | 2000/4 | 1.0 M LiTFSI in TEGDME | [121] |
| CoII-salen/MCNTs@MnO2 | 300/1000/500 | 3.7 | 13050 | 500/3.7 | 5 mM CoII-salen/1.0 M LiTFSI in TEGDME | [113] |
| MnO2/HPC | 300/1000/350 | 3.5 | ~9200 | 5000/- | 1.0 M LiTFSI in TEGDME | [118] |
| Holey 2-D Mn2O3 nanosheets | 33/1000/200 | 4.0 | 3600 | -/- | 1.0 M LiTFSI in TEGDME | [120] |
| CMO-4 (CoMn2O4) | 41/500/200 | ~4 | ~5860 | 400/- | 0.5 M LiTFSI in TEGDME | [107] |
| Co-CoO/N-CNR | 86/1000/100 | 3.9 | 10555 | 1000/- | 1.0 M LiTFSI in TEGDME | [111] |
| CoO mesoporous spheres | 300/1000/40 | ~3.6 | 4849 | -/- | 1.0 M LiTFSI in TEGDME | [97] |
| Co(OH)2@CNS | 40/715/150 | 3.8 | 5403 | -/- | 1.0 M LiCF3SO3 in TEGDME | [116] |
| CCO (Co2CrO4 yolk-shell nanospheres) | 236/1000/200 | 3.25 | 8554 | 500/3.85 | 1.0 M LiTFSI in TEGDME | [98] |
| p-CNT/Co3O4[M] | 116/500/200 | 3.65 | 4331 | 1100/4.35 | 1.0 M LiCF3SO3 in TEGDME | [93] |
| Co3O4-TiO2(B) | 200/1000/100 | 3.8 | 11000 | 500/ | 1.0 M LiTFSI in TEGDME | [101] |
| NCO@N-rGO (NiCo2O4 nanoparticles) | 112/1000/200 | 3.44 | 6716 | 1000/~3.9 | 1.0 M LiTFSI in TEGDME | [110] |
| Ni-MOFs | 170/600/1200 | ~3.7 | 9000 | 1440/~3.95 | 1.0 M LiTFSI in TEGDME | [96] |
| NiFeOx/CNT | 126/1000/200 | ~3.75 | 16987 | 2000/- | 0.5 M LiTFSI in TEGDME | [103] |
| Fe3O4-Fe nanohybrids | 100/1000/500 | ~3.87 | 13890 | 2000/~4.3 | 1.0 M LiNO3 in DMAc | [95] |
| 3DOM FePO4 | 300/1000/250 | 3.95 | 9923 | 500/~4.2 | 1.0 M LiTFSI in TEGDME | [124] |
| LFO-900 (α-Fe2O3-LaFeO3−x) | 108/500/100 | ~3.6 | 7183.3 | 500/- | 1.0 M LiTFSI in TEGDME | [106] |
| LNON/4 h (N-doped LaNiO3) | 50/500/250 | 3.9 | 5910 | 400/- | 1.0 M LiTFSI in TEGDME | [108] |
| G/meso-LaSrMnO | 50/500/125 | 3.4 | 6515 | 1000/~4.1 | 1.0 M LiTFSI in TEGDME | [112] |
| Red-CuGeO3-G (reduced CuGeO3-G) | 50/2000/1000 | 4.2 | 10030 | -/- | 1.0 M LiNO3 in DMAc | [126] |
| ZnO/VACNTs | 112/1000/50 | 3.55 | 4580 | -/- | 0.9 M LiTFSI in TEGDME | [104] |
| MoO2/graphitic foam | >55/500/1000 | ~4.18 | 5500 | 1000/~4.18 | 1.0 M LiTFSI in TEGDME | [115] |
| MoO3−x NSs-2 | 60/1000/100 | 3.45 | 10825 | -/- | 1.0 M LiClO4 in DMSO | [102] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Hou, Y.; Lu, Y.; Chen, J. Recent Progress on Catalysts for the Positive Electrode of Aprotic Lithium-Oxygen Batteries †. Inorganics 2019, 7, 69. https://doi.org/10.3390/inorganics7060069
Cai Y, Hou Y, Lu Y, Chen J. Recent Progress on Catalysts for the Positive Electrode of Aprotic Lithium-Oxygen Batteries †. Inorganics. 2019; 7(6):69. https://doi.org/10.3390/inorganics7060069
Chicago/Turabian StyleCai, Yichao, Yunpeng Hou, Yong Lu, and Jun Chen. 2019. "Recent Progress on Catalysts for the Positive Electrode of Aprotic Lithium-Oxygen Batteries †" Inorganics 7, no. 6: 69. https://doi.org/10.3390/inorganics7060069
APA StyleCai, Y., Hou, Y., Lu, Y., & Chen, J. (2019). Recent Progress on Catalysts for the Positive Electrode of Aprotic Lithium-Oxygen Batteries †. Inorganics, 7(6), 69. https://doi.org/10.3390/inorganics7060069