The Effect of Vanadium Inhalation on the Tumor Progression of Urethane-Induced Lung Adenomas in a Mice Model
Abstract
:1. Introduction
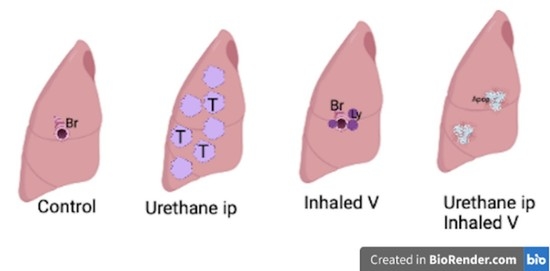
2. Results
2.1. Lung Histology
2.2. Proliferative Index
2.3. TUNEL Assay
3. Discussion
Aerosol Delivery
4. Materials and Methods
4.1. Animals
4.2. Experimental Protocol
4.3. Vanadium Exposure and Cardiothoracic Block Dissection
4.4. Tissue Sampling and Preparation
4.5. Immunohistochemistry for PCNA
4.6. TUNEL Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, W.J.; Meza, R.; Jeon, J.; Moolgavkar, S.H. Chapter 6: Lung cancer in never smokers: Epidemiology and risk prediction models. Risk Anal. 2012, 32 (Suppl. 1), S69–S84. [Google Scholar] [CrossRef] [Green Version]
- Green, L.S.; Fortoul, T.I.; Ponciano, G.; Robles, C.; Rivero, O. Bronchogenic cancer in patients under 40 years old. The experience of a Latin American country. Chest 1993, 104, 1477–1481. [Google Scholar] [CrossRef]
- Jamal-Hanjani, M.; Wilson, G.A.; McGranahan, N.; Birkbak, N.J.; Watkins, T.B.K.; Veeriah, S.; Shafi, S.; Johnson, D.H.; Mitter, R.; Rosenthal, R.; et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2109–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Palomo, G.; Rendon-Huerta, E.P.; Montano, L.F.; Fortoul, T.I. Vanadium compounds and cellular death mechanisms in the A549 cell line: The relevance of the compound valence. J. Appl. Toxicol. 2019, 39, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, G.M.; Karuri, S.W.; Zhang, H.; Zhang, L.; Khozin, S.; Kazandjian, D.; Tang, S.; Sridhara, R.; Keegan, P.; Pazdur, R. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J. Clin. Oncol. 2015, 33, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; Quezada, S.A.; Larkin, J.; Swanton, C. Translational implications of tumor heterogeneity. Clin. Cancer Res. 2015, 21, 1258–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, K.; Rozeboom, L.; Rivard, C.J.; Yu, H.; Ellison, K.; Melnick, M.A.C.; Hinz, T.K.; Chan, D.; Heasley, L.E.; Politi, K.; et al. Therapy-induced E-cadherin downregulation alters expression of programmed death ligand-1 in lung cancer cells. Lung Cancer 2017, 109, 1–8. [Google Scholar] [CrossRef]
- Kemp, C.J. Animal Models of Chemical Carcinogenesis: Driving Breakthroughs in Cancer Research for 100 Years. Cold Spring Harb. Protoc. 2015, 2015, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Gurley, K.E.; Moser, R.D.; Kemp, C.J. Induction of Lung Tumors in Mice with Urethane. Cold Spring Harb. Protoc. 2015, 2015, pdb–prot077446. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Alveno, R.A.; Faustino, C.B.; Correa, P.Y.; Vargas, C.M.; de Morais, J.; Rangel, M.P.; Velosa, A.P.; Fabro, A.T.; Teodoro, W.R.; et al. Intranasal Administration of Type V Collagen Reduces Lung Carcinogenesis through Increasing Endothelial and Epithelial Apoptosis in a Urethane-Induced Lung Tumor Model. Arch. Immunol. Ther. Exp. (Warsz) 2016, 64, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. The Evolution of Tumor Formation in Humans and Mice with Inherited Mutations in the p53 Gene. Curr. Top. Microbiol. Immunol. 2017, 407, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Petanidis, S.; Kioseoglou, E.; Hadzopoulou-Cladaras, M.; Salifoglou, A. Novel ternary vanadium-betaine-peroxido species suppresses H-ras and matrix metalloproteinase-2 expression by increasing reactive oxygen species-mediated apoptosis in cancer cells. Cancer Lett. 2013, 335, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozzo, C.; Sanna, D.; Garribba, E.; Serra, M.; Cantara, A.; Palmieri, G.; Pisano, M. Antitumoral effect of vanadium compounds in malignant melanoma cell lines. J. Inorg. Biochem. 2017, 174, 14–24. [Google Scholar] [CrossRef]
- Kowalski, S.; Hac, S.; Wyrzykowski, D.; Zauszkiewicz-Pawlak, A.; Inkielewicz-Stepniak, I. Selective cytotoxicity of vanadium complexes on human pancreatic ductal adenocarcinoma cell line by inducing necroptosis, apoptosis and mitotic catastrophe process. Oncotarget 2017, 8, 60324–60341. [Google Scholar] [CrossRef] [Green Version]
- Paredes Aller, S.; Quittner, A.L.; Salathe, M.A.; Schmid, A. Assessing effects of inhaled antibiotics in adults with non-cystic fibrosis bronchiectasis—Experiences from recent clinical trials. Expert Rev. Respir. Med. 2018, 12, 769–782. [Google Scholar] [CrossRef]
- Hamzawy, M.A.; Abo-Youssef, A.M.; Salem, H.F.; Mohammed, S.A. Antitumor activity of intratracheal inhalation of temozolomide (TMZ) loaded into gold nanoparticles and/or liposomes against urethane-induced lung cancer in BALB/c mice. Drug Deliv. 2017, 24, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Carafa, M.; Marianecci, C.; Donatella, P.; D’i Marzio, L.; Celia, M.; Fresta, F.; Alhaique, F. Novel concept in pulmonary delivery. In Chronic Obstructive Pulmonary Disease-Current Concept and Practice; Ong, K.C., Ed.; IntechOpen Limited: London, UK, 2012. [Google Scholar]
- Gagnadoux, F.; Pape, A.L.; Lemarie, E.; Lerondel, S.; Valo, I.; Leblond, V.; Racineux, J.L.; Urban, T. Aerosol delivery of chemotherapy in an orthotopic model of lung cancer. Eur. Respir. J. 2005, 26, 657–661. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. Inhaled Corticosteroids. Pharmaceuticals 2010, 3, 514–540. [Google Scholar] [CrossRef] [Green Version]
- Borghardt, J.M.; Kloft, C.; Sharma, A. Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes. Can. Respir. J. 2018, 2018, 2732017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopf-Maier, P.; Wagner, W.; Hesse, B.; Kopf, H. Tumor inhibition by metallocenes: Activity against leukemias and detection of the systemic effect. Eur. J. Cancer 1981, 17, 665–669. [Google Scholar] [CrossRef]
- Thompson, H.J.; Chasteen, N.D.; Meeker, L.D. Dietary vanadyl(IV) sulfate inhibits chemically-induced mammary carcinogenesis. Carcinogenesis 1984, 5, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Kopf-Maier, P.; Wagner, W.; Liss, E. Induction of cell arrest at G1/S and in G2 after treatment of Ehrlich ascites tumor cells with metallocene dichlorides and cis-platinum in vitro. J. Cancer Res. Clin. Oncol. 1983, 106, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Oinam, S.; Basu, M.; Chatterjee, M. Vanadium chemoprevention of 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis: Probable involvement of representative hepatic phase I and II xenobiotic metabolizing enzymes. Breast Cancer Res. Treat 2000, 63, 133–145. [Google Scholar] [CrossRef]
- Sankar Ray, R.; Roy, S.; Ghosh, S.; Kumar, M.; Chatterjee, M. Suppression of cell proliferation, DNA protein cross-links, and induction of apoptosis by vanadium in chemical rat mammary carcinogenesis. Biochim. Biophys. Acta 2004, 1675, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, A.M. Vanadium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 249–265. [Google Scholar] [CrossRef]
- Mateos-Nava, R.A.; Rodriguez-Mercado, J.J.; Altamirano-Lozano, M.A. Premature chromatid separation and altered proliferation of human leukocytes treated with vanadium (III) oxide. Drug Chem. Toxicol. 2017, 40, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Leon, I.E.; Porro, V.; Di Virgilio, A.L.; Naso, L.G.; Williams, P.A.; Bollati-Fogolin, M.; Etcheverry, S.B. Antiproliferative and apoptosis-inducing activity of an oxidovanadium(IV) complex with the flavonoid silibinin against osteosarcoma cells. J. Biol. Inorg. Chem. 2014, 19, 59–74. [Google Scholar] [CrossRef]
- Lu, L.P.; Suo, F.Z.; Feng, Y.L.; Song, L.-L.; Li, Y.; Li, Y.-J.; Wang, K.-T. Synthesis and biological evaluation of vanadium complexes as novel anti-tumor agents. Eur. J. Med. Chem. 2019, 176, 1–10. [Google Scholar] [CrossRef]
- Xi, W.S.; Tang, H.; Liu, Y.Y.; Liu, C.Y.; Gao, Y.; Cao, A.; Liu, Y.F.; Chen, Z. Cytotoxicity of vanadium oxide nanoparticles and titanium dioxide-coated vanadium oxide nanoparticles to human lung cells. J. Appl. Toxicol. 2020, 40, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Dai, C.L.; Liang, Z.; Run, X.; Iqbal, K.; Liu, F.; Gong, C.X. Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Abeta level and microglia activation in the brains of 3xTg-AD mice. Exp. Neurol. 2014, 261, 610–619. [Google Scholar] [CrossRef]
- Gagnadoux, F.; Hureaux, J.; Vecellio, L.; Urban, T.; Le Pape, A.; Valo, I.; Montharu, J.; Leblond, V.; Boisdron-Celle, M.; Lerondel, S.; et al. Aerosolized chemotherapy. J. Aerosol. Med. Pulm. Drug Deliv. 2008, 21, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, H.M.; Elzoghby, A.O.; Helmy, M.W.; Abdelfattah, E.A.; Fang, J.Y.; Samaha, M.W.; Freag, M.S. Inhalable Lactoferrin/Chondroitin-Functionalized Monoolein Nanocomposites for Localized Lung Cancer Targeting. ACS Biomater. Sci. Eng. 2020, 6, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Fortoul, T.I.; Rojas-Lemus, M.; Rodriguez-Lara, V.; Gonzalez-Villalva, A.; Ustarroz-Cano, M.; Cano-Gutierrez, G.; Gonzalez-Rendon, S.E.; Montano, L.F.; Altamirano-Lozano, M. Overview of environmental and occupational vanadium exposure and associated health outcomes: An article based on a presentation at the 8th International Symposium on Vanadium Chemistry, Biological Chemistry, and Toxicology, Washington DC, August 15–18, 2012. J. Immunotoxicol. 2014, 11, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Roomi, N.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Chemopreventive Effect of a Novel Nutrient Mixture on Lung Tumorigenesis Induced by Urethane in Male A/J Mice. Tumori. J. 2009, 95, 508–513. [Google Scholar] [CrossRef]
- Fortoul, T.I.; Soto-Mota, A.; Rojas-Lemus, M.; Rodriguez-Lara, V.; Gonzalez-Villalva, A.; Montano, L.F.; Paez, A.; Colin-Barenque, L.; Lopez-Valdez, N.; Cano-Gutierrez, G.; et al. Myocardial connexin-43 and N-Cadherin decrease during vanadium inhalation. Histol. Histopathol. 2016, 31, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Costigan, M.C.; Dobson, R. Vanadium Pentoxide and Other Inorganic Vanadium Compounds; World Health Organization & International Programme on Chemical Safety 2001; World Health Organization: Geneva, Switzerland, 2001; Available online: https://apps.who.int/iris/handle/10665/42365 (accessed on 20 August 2021).
- Chieco, P.; Derenzini, M. The Feulgen reaction 75 years on. Histochem. Cell Biol. 1999, 111, 345–358. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Valdez, N.; Rojas-Lemus, M.; Fortoul, T.I. The Effect of Vanadium Inhalation on the Tumor Progression of Urethane-Induced Lung Adenomas in a Mice Model. Inorganics 2021, 9, 78. https://doi.org/10.3390/inorganics9110078
López-Valdez N, Rojas-Lemus M, Fortoul TI. The Effect of Vanadium Inhalation on the Tumor Progression of Urethane-Induced Lung Adenomas in a Mice Model. Inorganics. 2021; 9(11):78. https://doi.org/10.3390/inorganics9110078
Chicago/Turabian StyleLópez-Valdez, Nelly, Marcela Rojas-Lemus, and Teresa I. Fortoul. 2021. "The Effect of Vanadium Inhalation on the Tumor Progression of Urethane-Induced Lung Adenomas in a Mice Model" Inorganics 9, no. 11: 78. https://doi.org/10.3390/inorganics9110078
APA StyleLópez-Valdez, N., Rojas-Lemus, M., & Fortoul, T. I. (2021). The Effect of Vanadium Inhalation on the Tumor Progression of Urethane-Induced Lung Adenomas in a Mice Model. Inorganics, 9(11), 78. https://doi.org/10.3390/inorganics9110078