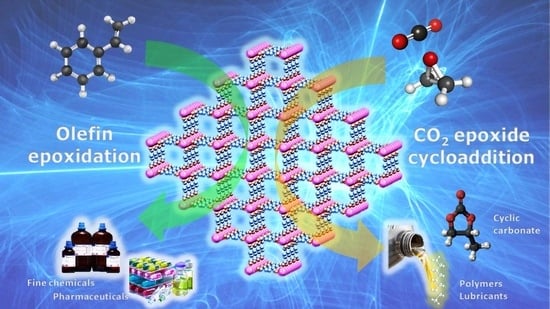
Metal Organic Frameworks as Heterogeneous Catalysts in Olefin Epoxidation and Carbon Dioxide Cycloaddition
Abstract
:1. Introduction
2. Olefin Epoxidation
2.1. Metal Nodes/Clusters as Catalytically Active Sites
| MOF | Substrate | Reaction Data T (°C) P (atm) Time (h) | Oxidant/Cocatalyst/ Solvent a | Conversion % | Epoxide Selectivity% | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Co6-MOF-3 | Styrene | 100 | 1 | 14 | Air/-/DMF | 99 | 90 | [29] |
| Co-MOF-150-2 | α-Pinene | 90 | 1 | 5 | Air/CHP/- | 99.5 | 96.2 | [31] |
| Cyclooctene | 90 | 1 | 5 | Air/CHP/- | 78.5 | - | [31] | |
| 1-Decene | 90 | 1 | 5 | Air/CHP/- | 87.2 | - | [31] | |
| NTUZ30 | trans-Stilbene | 100 | 7 | 1 | O2/-/- | 98.2 | 95.6 | [32] |
| (NH4)2[Co3(Ina)(BDC)3(HCOO)] | Cyclooctene | 35 | 1.5 | 1 | IBA/-/CH3CN | 98 | 92 | [33] |
| Cu3(BTC)2 | Cyclooctene | 75 | 1 | 24 | Air/TBHP/Toluene | 20 | - | [36] |
| 1-Hexene | 25 | 1 | Air/TBHP/Toluene | 30.5 | - | [36] | ||
| Cu-MOF nanosheets | Cyclooctene | 25 | 1 | 12 | O2/-/CH3CN | 100 | - | [37] |
| 1-Hexene | 25 | 1 | O2/-/CH3CN | 67.2 | - | [37] | ||
| Cu4O(OH)2(Me2trz-pba)4 | Cyclooctene | 75 | 1 | 24 | Air/TBHP/Toluene | 90 | 80 | [36] |
| Cu(Me-4py-trz-ia) | Cyclooctene | 75 | 1 | 24 | Air/TBHP/Toluene | 38 | 60 | [36] |
| {[Cu(L3-H)(DMA)]·DMA·2H2O}∞ | cis-Stilbene | 60 | 1 | 24 | t-BuOOH/-/CH3CN | 94.4 | - | [38] |
| Cyclooctene | 60 | 1 | 24 | t-BuOOH/-/CH3CN | 98 | - | [38] | |
| {2(Him)·[Cu(pdc)2]}n | Cyclohexene | 60 | 1 | 8 | Air/H2O2/EtOH | 100 | - | [38] |
| Cyclooctene | 60 | 1 | 8 | Air/H2O2/EtOH | 100 | - | [38] | |
| [Cu3(L4)3(H2O)2(DMF)]n | Styrene | 40 | 1 | 6 | O2/TMA/CH3CN | 90 | 88 | [39] |
| Cyclooctene | 40 | 1 | 6 | O2/TMA/CH3CN | 99 | 99 | [39] | |
| {(Co(L2)H2O))2·H2O)}n | Cyclohexene | 60 | 1 | 6 | t-BuOOH/-/- | 82.56 | 71.93 | [30] |
| UiO-66 | 2-Cyclohexen-1-one | 70 | 1 | 1 | H2O2/-/CH3CN | 20 | 60 | [46] |
| 2-Cyclohexen-1-one | 70 | 1 | 2 | H2O2/-/EtOAc | 18 | 45 | [46] | |
| Chalcone | 70 | 1 | 0.5 | H2O2/-/EtOAc | 30 | 50 | [46] | |
| UiO-67 | 2-Cyclohexen-1-one | 70 | 1 | 1 | H2O2/-/CH3CN | 20 | 55 | [46] |
| 2-Cyclohexen-1-one | 70 | 1 | 2 | H2O2/-/EtOAC | 20 | 55 | [46] | |
| Chalcone | 70 | 1 | 0.5 | H2O2/-/CH3CN | 40 | 40 | [46] | |
2.2. Mixed-Metal Species
| MOF | Substrate | Reaction Data T (°C) P (atm) Time (h) | Oxidant/Cocatalyst/Solvent a | Conversion % | Epoxide Selectivity% | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Mn0.1Cu0.9-MOF | Styrene | 0 | 1 | 6 | H2O2/-/DMF | 90.2 | 94.3 | [49] |
| Cu0.25-Co0.75-MOF | Styrene | 80 | 1 | 8 | Air/TBHP/t-BuOH/H2O2 | 97.81 | 83.4 | [48] |
| Zn1Co1-ZIF | Styrene | 100 | 1 | 24 | TBHP/-/DMF | 99 | 71.31 | [56] |
| LaCoODA | Cyclohexene | 75 | 1 | 24 | O2 flow/-/- | 85 | 75 | [50] |
| LaCuODA | Cyclohexene | 75 | 1 | 24 | O2 flow/-/- | 67 | 55 | [50] |
| NENU-MV-1 | Cyclohexene | 35 | 1 | 4 | Air/IBA/CH3CN | 95 | 86 | [55] |
| NU-1000-Fe-Cl | Cyclohexene | 120 | 0.03 | 3 | H2O2/-/- | - | 70 | [52] |
| NU-1000-Fe-Cl | Cyclohexene | 120 | 0.03 | 3 | H2O2/-/- | - | 70 | [52] |
2.3. Organic Linkers with Functional Catalytically Active Sites
3. Epoxidation with MOF-Based Composites
| MOF | Substrate | Reaction Data T (°C) P (atm) Time (h) | Oxidant/Cocatalyst/Solvent a | Conversion % | Epoxide Selectivity% | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| DPSNs@Cu-BTC | Cyclooctene | 40 | 1 | 4 | O2/TMA/CH3CN | 99 | 99 | [75] |
| Styrene | 40 | 1 | 6 | O2/TMA/CH3CN | 62 | 65 | [75] | |
| Fe3O4@P4VP@ZIF-8 | Cyclohexene Cyclooctene Norbornene | 60 | 1 | 12 | O2/TMA/CH3CN | 99 | 99 | [90] |
| Fe3O4/Cu3(BTC)2 | Cyclohexene Cyclooctene Norbornene | 40 | 1 | 6–8 | O2/IBA/CH3CN | 99 | 99 | [88] |
| Styrene | 40 | 1 | 6–8 | O2/IBA/CH3CN | 99 | 84 | [88] | |
| PCN-222(Fe)@Zr-BPDC(UiO-67) | 1-Hexene | r.t | 1 | 12 | PhIO/-/CH3CN | 99 | - | [91] |
| Cyclopentene | r.t | 1 | 12 | PhIO/-/CH3CN | 99 | - | [91] | |
| Cyclohexene | r.t | 1 | 12 | PhIO/-/CH3CN | 99 | - | [91] | |
| CoPMA@UiO-bpy | Cyclooctene | 70 | 1 | 6 | H2O2/-/CH3CN | 91 | 99 | [80] |
| Styrene | 80 | 1 | 6 | O2/t-BuOOH/- | 80 | 56 | [80] | |
| PMo10V2-ILs@MIL-100(Fe) | Cyclohexene | 60 | 1 | 4 | H2O2/-/CH3CN | 92 | 93 | [91] |
| [Cu6(bip)12(PMoVI12O40)2(PMoVMoVI11O40O2)]·8H2O | Cyclooctene | 20 | 1 | 4 | H2O2/tBuOH/CH3CN | >99 | 74.1 | [84] |
| 1−Hexene | 20 | 1 | 4 | H2O2/tBuOH/CH3CN | >99 | 91.9 | [84] | |
| 1−Octene | 20 | 1 | 4 | H2O2/tBuOH/CH3CN | >99 | 71.5 | [84] | |
| Pd/UiO-66-NH2 | Styrene | 80 | 1 | 12 | N2/TBHP/CH3CN | 90.8 | 96.5 | [85] |
| [Co3IICo2III(H2bib)2(Hbib)2(PW9O34)2(H2O)6] ·6H2O | Cyclohexene | 20 | 1 | 4 | H2O2/tBuOH/CH3CN | 72.9 | 95.3 | [84] |
| 1−Hexene | 20 | 1 | 4 | H2O2/tBuOH/CH3CN | >99 | 85.9 | [84] | |
| 1−Octene | 20 | 1 | 4 | H2O2/tBuOH/CH3CN | 95.5 | 70.1 | [84] | |
| POM/MIL-100(Fe) | 3Z,6Z,9Z-Octadecatriene | 40 | 1 | 24 | H2O2/-/CH3CN | 30 | 82 | [83] |
| MIL-101-GH-TS-1 | Octane | 40 | 1 | 12 | H2O2 (30%)/-/- | 15 | - | [77] |
| Pd@UiO-66-sal(Mo) | cis-Cyclooctene | r.t | 1 | 6 | H2O2/CH3OH/H2O | - | - | [86] |
4. CO2 Epoxide Cycloaddition to Cyclic Carbonates
| MOF | Substrate | Reaction Data T (°C) CO2 P (atm) Time (h) | Cocatalys a | Conversion % | Cyclic Carbonate Selectivity% | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Hf-NU-1000 | Styrene epoxide | r.t. | 1 | 56 | TBAB | 100 | 100 | [101] |
| Propylene oxide | r.t. | 1 | 26 | TBAB | 100 | 100 | [101] | |
| Epoxide divinylbenzene dioxide | r.t. | 1 | 19 | TBAB | 100 | 100 | [101] | |
| PCN-224-Mn(tart) | Styrene epoxide | 60 | 1 | 15 | TBAB | 96 94 ee (S) | 100 | [71] |
| (2,3-Epoxypropyl)benzene | 60 | 1 | 15 | TBAB | 87 90 ee (S) | 100 | [71] | |
| Propylene oxide | 60 | 1 | 15 | TBAB | 99 98 ee (S) | 100 | [71] | |
| 1,2-Epoxybutane | 60 | 1 | 15 | TBAB | 91 97 ee (S) | 100 | [71] | |
| 1,2-Epoxyoctane | 60 | 1 | 15 | TBAB | 78 96 ee | 100 | [71] | |
| polyILs@MIL-101(Cr) | 1-Butene oxide | 45 | 1 | 48 | - | 94 | 100 | [110] |
| 1,2-Epoxyhexane | 70 | 1 | 24 | - | 89 | 100 | [110] | |
| 3-Hydroxy-1,2-epoxypropane | 70 | 1 | 24 | - | >99 | 100 | [110] | |
| 1,2-Epoxy-3-phenoxypropane | 70 | 1 | 24 | - | 95 | 100 | [110] | |
| NH2-MIL-101(Al) | Styrene oxide | 120 | 18 | 6 | TBAB | 93.6 | 99 | [115] |
| UiO-66-NH2 | Epoxy fatty acid methyl ester | 120 | 30 | 12 | TBAB | 94 | 80 | [117] |
| ILB@U6N | Epichlorohydrin | 80 | 118 | 4 | - | 94 | 99 | [109] |
| (I−)Meim-UiO-66 | Epichlorohydrin | 120 | 1 | 24 | - | 100 | 93 | [107] |
| VPI-100 (Ni) | Epichlorohydrin | 90 | 10 | 6 | TBAB | 96 | - | [112] |
| VPI-100 (Cu) | Epichlorohydrin | 90 | 10 | 6 | TBAB | 94 | - | [112] |
| [Zn4OL43]n | Epichlorohydrin | 50 | 1 | 4 | - | 96 | 99 | [107] |
| 2-Vinyloxirane | 50 | 1 | 4 | - | 99 | 81 | [107] | |
| Hf-Bipy-UiO-67(Mn(OAc)2 | Epichlorohydrin | 25 | 1 | 12 | TBAB | 83.2 | 99 | [119] |
| Zn-MOF-184 | Styrene oxide | 80 | 1 | 6 | TBAB | 96 | 85 | [102] |
| Propylene oxide | 80 | 1 | 6 | TBAB | 100 | 75 | [102] | |
| Epichlorohydrin | 80 | 1 | 6 | TBAB | 100 | 70 | [102] | |
| Cyclohexene oxide | 80 | 1 | 6 | TBAB | 69 | 85 | [102] | |
| SALI-4-Py-I-(Zr), | Styrene oxide | 80 | 4 | 4 | - | 99 | 98 | [111] |
| ILA@U6N | Epichlorohydrin | 80 | 118 | 4 | - | 65 | 99 | [109] |
| UiO-67-IL | Epichlorohydrin | 90 | 1 | 3 | TBAB | 99 | 100 | [108] |
| Epichlorohydrin | 90 | 1 | 3 | - | 99 | 96 | [108] | |
| {[Zn2(TBIB)2(HTCPB)2]·9DMF·19H2O}n | Epichlorohydrin | r.t | 1 | 24 | TBAB | 99 | 100 | [105] |
| ZIF-95 | Propylene oxide | 120 | 118 | 24 | TBAB | 91 | 99 | [106] |
| ZSF-1 | Styrene oxide | 100 | 1 | 20 | TBAB | 93 | - | [120] |
| Epichlorohydrin | 100 | 1 | 20 | TBAB | 99 | - | [120] | |
| Zn(3,5-NH2-Bpz) | Epichlorohydrin | 120 | 5 | 24 | - | 98 | 50 | [118] |
| Zn(BPZNH2) | Epichlorohydrin | 120 | 5 | 24 | - | 96 | 33 | [118] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MOFs | |
| Co6-MOF-3 | [(Co6(OH)6(TCA)2(BPB)3]n |
| Co-MOF-150-2 | [Co(BDC)]n |
| Cu0.25-Co0.75-MOF | [(Cu0.25-Co0.75)3(BTC)2]n |
| HKUST-1 | [Cu3(BTC)2]n |
| LaCoODa | {[La2Co3(ODA)6(H2O)6]·12H2O}n |
| LaCuODA | {[La2Cu3(µ-H2O)(ODA)6(H2O)3]∙3H2O}n |
| MIL-100(Fe) | [Fe3O(OH)(H2O)2(BDC)3]n |
| MIL-101 | [Cr3O(H2O)2F(BDC)]n |
| Mn0.1Cu0.9-MOF | [(Mn0.1-Cu0.9)3(BTC)2]n |
| NENU-MV-1 | {[Ni(4,4′-bpy)2]2[V7IVV9VO38Cl]·(4,4′-bpy)·6H2O}n |
| NH2-MIL-101(Al) | [Al3O(OH)(H2O)2(BDCNH2)3]n |
| NH2-MIL-101(Cr) | Cr3O(H2O)2F(NH2-BDC) |
| NTUZ30 | {[Co3(μ3-OH)(HBTC)(BTC)2Co(HBTC)]·(HTEA)3·H2O}n |
| PCN-222 | [Zr6(μ3-OH)8(OH)8-(TCPP)2]n |
| PCN-224 | [Zr6(μ3-OH)12(OH)16-(TCPP)1.5]n |
| TMU-16-NH2 | {[Zn2(NH2-BDC)2(4-bpdh)]·3DMF}n |
| UiO-66 | [Zr6O4(OH)4(BDC)6]n |
| UiO-66-NH2 | [Zr6O4(OH)4(NH2-BDC)6]n |
| UiO-67 | [Zr6(μ3-O)4(μ3-OH)4(BPDC)6]n |
| UiO-68 | [Zr6(μ3-O)4(μ3-OH)4(TPDC)6]n |
| UiO-bpy | [Zr6O4(OH)4(bpy)6]n |
| VPI-100(Cu) | [Zr6(μ3-OH)8(OH)8(Cu-L1)4]n |
| VPI-100(Ni) | [Zr6(μ3-OH)8(OH)8(Ni-L1)4]n |
| ZIF-67 | [Co(MeIm)2]n |
| ZIF-8 | [Zn(MeIm)2]n |
| ZIF-95 | [Zn(cbIm)2]n |
| Zn-MOF-184 | [Zn2(EDOB)]n |
| Zr-NU-1000 | ([Zr6(μ3-O)4(μ3-OH)4(OH)4(H2O)4(TBAPy)2]n |
| ZSF-1 | [Zr6O4(OH)4(metallosalen)6]n |
| Hf-NU-1000 | [(Hf6(μ3-O)4(μ3-OH)4(OH)4(OH2)4(TBAPy)2]n |
Appendix A. Chart of the MOF Linkers Present in This Review and Their Relative Abbreviations
| Structural Formula | Name | Abbreviation |
 | 1-(4-Cyanobenzyl)-5-methyl-1H-imidazole | cbIm |
 | 1,3,5-tri(1H-Benzo[d]imidazol-1-yl)benzene | TBIB |
 | 1,3,5-tris(4′-Carboxy-phenyl-)benzene | H3TCPB |
 | 1,3,6,8-(p-Benzoate)pyrene | H4TBAPy |
 | 1,3-bis(Imidazolyl)propane | H2bip |
 | 1,4-bis(Imidazol)butane | bib |
 | 2,2′,6,6′-Tetramethoxy-4,4′-biphenyldicarboxylic acid | H2L4 |
 | 2,2-Bipyridine-4,4′-dicarboxylic acid | H2Bpy |
 | 2,5-bis(4-Pyridyl)-3,4-diaza-2,4-hexadiene | 4-bpdh |
 | 2-Aminoterephthalic acid | H2BDCNH2 |
 | 2-Methylimidazole | HmeIm |
 | 2-Picolinic acid | PCA |
 | 3,5-Diamino-4,4′-bipyrazole | H2-NH2-Bpz |
 | 4-((1-Carboxy-2-(1H-imidazol-4-yl)ethylamino)methyl)benzoic acid | H2L2 |
 | 4-(3,5-Dimethyl-4-H-1,2,4-triazol-4-yl) benzoate | Me2trz-pba |
 | 4,4′-Bipyridine | 4,4′-bipy |
 | 4,4′-(Ethyne-1,2-diyl)bis(2-hydroxybenzoic acid) | H4EDOB |
 | 4,4′,4′′-Tricarboxyltriphenylamine | H3TCA |
 | 5-(3-Methyl-5-(pyridine-4-yl)-4H-1,2,4-triazol-4-yl) isophthalate | Me-4py-trz-ia |
 | 5,10,15,20-Tetrakis(4-carboxyphenyl)porphyrin | H6TCPP |
 | 5-Dihydroxyterephthalic acid | H4DHTA |
 | 6,13-Dicarboxy-1,4,8,11-tetraazacyclotetradecane | L1 |
 | Benzene-1,3-5 tricarboxylic acid | H3BTC |
 | Biphenyl-4,4′-dicarboxylic acid | H2BPDC |
 | Imidazole | HIm |
 | Isonicotinate | Ina |
 | N,N′-bis(3-tert-Butyl-5-(carboxy)salicylide | H4L5 |
 | Oxydiacetic acid | H2ODA |
 | Pyridine-2,5-dicarboxylic acid | H2pdc |
 | Pyridine-2-aldehyde | PI |
 | Salicylaldehyde | SA |
 | Thiophene-2-carbaldehyde | TC |
 | Triethylamine | TEA |
 | Tris(4′-carboxybiphenyl)amine | H3L3 |
References
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Pettinari, C.; Marchetti, F.; Mosca, N.; Drozdov, A. Application of metal-organic frameworks. Polym. Int. 2017, 66, 93. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nat. Cell Biol. 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Schaus, S.E.; Brandes, B.D.; Larrow, J.F.; Tokunaga, M.; Hansen, K.B.; Gould, A.E.; Furrow, M.E.; Jacobsen, E.N. Highly Selective Hydrolytic Kinetic Resolution of Terminal Epoxides Catalyzed by Chiral (salen)CoIII Complexes. Practical Synthesis of Enantioenriched Terminal Epoxides and 1,2-Diols. J. Am. Chem. Soc. 2002, 124, 1307. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2011, 112, 933. [Google Scholar] [CrossRef]
- Campagnol, N.; Souza, E.R.; De Vos, D.E.; Binnemans, K.; Fransaer, J. Luminescent terbium-containing metal–organic framework films: New approaches for the electrochemical synthesis and application as detectors for explosives. Chem. Commun. 2014, 50, 12545–12547. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bo, X.; Nsabimana, A.; Han, C.; Li, M.; Guo, L. Electrocatalytically active cobalt-based metal–organic framework with incorporated macroporous carbon composite for electrochemical applications. J. Mater. Chem. A 2014, 3, 732. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Junk, P.C. Rapid mechanochemical synthesis of two new Cd(ii)-based metal–organic frameworks with high removal efficiency of Congo red. CrystEngComm 2014, 17, 686. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Chao, T.-C.; Zhong, M.-S. Influence of Counteranions on the Structural Modulation of Silver–Di(3-pyridylmethyl)amine Coordination Polymers. Cryst. Growth Des. 2013, 13, 2953. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11. [Google Scholar] [CrossRef]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef]
- Murray, J.L.; Mircea, D.; Long, R.J. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett. 2020, 12, 103. [Google Scholar] [CrossRef]
- Wang, H.-S. Metal–organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Gao, X.; Dong, Y.; Li, S.; Zhou, J.; Wang, L.; Wang, B. MOFs and COFs for Batteries and Supercapacitors. Electrochem. Energy Rev. 2019, 3, 81–126. [Google Scholar] [CrossRef]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Yaghi, O.M. Metal–Organic Frameworks for Water Harvesting from Air, Anywhere, Anytime. ACS Central Sci. 2020, 6, 1348–1354. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; He, H.; Yang, Y.; Cui, Y.; Qian, G. Structural Variation and Switchable Nonlinear Optical Behavior of Metal–Organic Frameworks. Small 2021, 17, 2006649. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Opanasenko, M.; Čejka, J.; Garcia, H. Metal organic frameworks as heterogeneous catalysts for the production of fine chemicals. Catal. Sci. Technol. 2013, 3, 2509–2540. [Google Scholar] [CrossRef]
- Vanesa, C.-C.; Rosa, M.M.-A. Advances in Metal-Organic Frameworks for Heterogeneous Catalysis. Recent Pat. Chem. Eng. 2011, 4, 1–16. [Google Scholar]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-Organic Frameworks: Opportunities for Catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, S. Biomimetic catalysis of metal–organic frameworks. Dalt. Trans. 2016, 45, 9744–9753. [Google Scholar] [CrossRef]
- Corma, A.; García, H. Lewis Acids as Catalysts in Oxidation Reactions: From Homogeneous to Heterogeneous Systems. Chem. Rev. 2002, 102, 3837–3892. [Google Scholar] [CrossRef]
- Kravchenko, D.E.; Tyablikov, I.A.; Kots, P.A.; Kolozhvari, B.A.; Fedosov, D.A.; Ivanova, I.I. Olefin Epoxidation over Metal-Organic Frameworks Modified with Transition Metals. Pet. Chem. 2019, 58, 1255–1262. [Google Scholar] [CrossRef]
- Arai, H.; Uehara, K.; Kinoshita, S.I.; Kunugi, T. Olefin Oxidation-Mercuric Salt-Active Charcoal Catalysis. Ind. Eng. Chem. Prod. Res. Dev. 1972, 11, 308–312. [Google Scholar] [CrossRef]
- Liniger, M.; Liu, Y.; Stoltz, B.M. Sequential Ruthenium Catalysis for Olefin Isomerization and Oxidation: Application to the Synthesis of Unusual Amino Acids. J. Am. Chem. Soc. 2017, 139, 13944–13949. [Google Scholar] [CrossRef]
- Gao, J.; Bai, L.; Zhang, Q.; Li, Y.; Rakesh, G.; Lee, J.-M.; Yang, Y.; Zhang, Q. Co6(μ3-OH)6 cluster based coordination polymer as an effective heterogeneous catalyst for aerobic epoxidation of alkenes. Dalton Trans. 2014, 43, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.-Z.; Jin, Y.-Q.; Geng, L.; Zhang, D.-S.; Hu, H.; Li, T.; Wang, B.; Li, J.-R. Pillar-Layered Metal–Organic Frameworks Based on a Hexaprismane [Co6(μ3-OH)6] Cluster: Structural Modulation and Catalytic Performance in Aerobic Oxidation Reaction. Inorg. Chem. 2020, 59, 11728–11735. [Google Scholar] [CrossRef]
- Yu, F.; Xiong, X.; Huang, K.; Zhou, Y.; Li, B. 2D Co-based coordination polymer with a histidine derivative as an efficient heterogeneous catalyst for the oxidation of cyclohexene. CrystEngComm 2017, 19, 2126–2132. [Google Scholar] [CrossRef]
- Zhang, H.; He, J.; Lu, X.; Yang, L.; Wang, C.; Yue, F.; Zhou, D.; Xia, Q. Fast-synthesis and catalytic property of heterogeneous Co-MOF catalysts for the epoxidation of α-pinene with air. New J. Chem. 2020, 44, 17413–17421. [Google Scholar] [CrossRef]
- Lu, H.-S.; Bai, L.; Xiong, W.-W.; Li, P.; Ding, J.; Zhang, G.; Wu, T.; Zhao, Y.; Lee, J.-M.; Yang, Y.; et al. Surfactant Media To Grow New Crystalline Cobalt 1,3,5-Benzenetricarboxylate Metal–Organic Frameworks. Inorg. Chem. 2014, 53, 8529–8537. [Google Scholar] [CrossRef]
- Sha, S.; Yang, H.; Li, J.; Zhuang, C.; Gao, S.; Liu, S. Co(II) coordinated metal-organic framework: An efficient catalyst for heterogeneous aerobic olefins epoxidation. Catal. Commun. 2014, 43, 146–150. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Qin, M.; Huang, R.; Li, Z. Cu(ii)-and Co(ii)-containing metal–organic frameworks (MOFs) as catalysts for cyclohexene oxidation with oxygen under solvent-free conditions. RSC Adv. 2012, 2, 3309–3314. [Google Scholar] [CrossRef]
- Lashanizadegan, M.; Ashari, H.A.; Sarkheil, M.; Anafcheh, M.; Jahangiry, S. New Cu(II), Co(II) and Ni(II) azo-Schiff base complexes: Synthesis, characterization, catalytic oxidation of alkenes and DFT study. Polyhedron 2021, 200, 115148. [Google Scholar] [CrossRef]
- Junghans, U.; Suttkus, C.; Lincke, J.; Lässig, D.; Krautscheid, H.; Gläser, R. Selective oxidation of cyclooctene over copper-containing metal-organic frameworks. Microporous Mesoporous Mater. 2015, 216, 151–160. [Google Scholar] [CrossRef]
- Wang, B.; Jin, J.; Ding, B.; Han, X.; Han, A.; Liu, J. General Approach to Metal-Organic Framework Nanosheets With Controllable Thickness by Using Metal Hydroxides as Precursors. Front. Mater. 2020, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Ren, Y.; Jiang, H.; Cai, B.; Lu, J. Synthesis, Structures, and Properties of Two Three-Dimensional Metal–Organic Frameworks, Based on Concurrent Ligand Extension. Inorg. Chem. 2012, 51, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, Y.; Qi, C.; Jiang, H. Fully meta-Substituted 4,4′-Biphenyldicarboxylate-Based Metal–Organic Frameworks: Synthesis, Structures, and Catalytic Activities. Eur. J. Inorg. Chem. 2017, 11, 1478–1487. [Google Scholar] [CrossRef]
- Saha, D.; Gayen, S.; Koner, S. Cu(II)/Cu(II)-Mg(II) containing pyridine-2,5-dicarboxylate frameworks: Synthesis, structural diversity, inter-conversion and heterogeneous catalytic epoxidation. Polyhedron 2018, 146, 93–98. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Portillo, A.S.; Asiri, A.M.; Garcia, H. Engineering UiO-66 Metal Organic Framework for Heterogeneous Catalysis. ChemCatChem 2019, 11, 899–923. [Google Scholar] [CrossRef]
- Vandichel, M.; Hajek, J.; Vermoortele, F.; Waroquier, M.; De Vos, D.E.; Van Speybroeck, V. Active site engineering in UiO-66 type metal–organic frameworks by intentional creation of defects: A theoretical rationalization. CrystEngComm 2014, 17, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Arrozi, U.S.; Wijaya, H.W.; Patah, A.; Permana, Y. Efficient acetalization of benzaldehydes using UiO-66 and UiO-67: Substrates accessibility or Lewis acidity of zirconium. Appl. Catal. A Gen. 2015, 506, 77–84. [Google Scholar] [CrossRef]
- Zheng, H.-Q.; Zeng, Y.-N.; Chen, J.; Lin, R.-G.; Zhuang, W.-E.; Cao, R.; Lin, Z.-J. Zr-Based Metal–Organic Frameworks with Intrinsic Peroxidase-Like Activity for Ultradeep Oxidative Desulfurization: Mechanism of H2O2 Decomposition. Inorg. Chem. 2019, 58, 6983–6992. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Klet, R.C.; Hupp, J.T.; Farha, O. Probing the correlations between the defects in metal–organic frameworks and their catalytic activity by an epoxide ring-opening reaction. Chem. Commun. 2016, 52, 7806–7809. [Google Scholar] [CrossRef] [PubMed]
- Zalomaeva, O.V.; Evtushok, V.Y.; Ivanchikova, I.D.; Glazneva, T.S.; Chesalov, Y.A.; Larionov, K.P.; Skobelev, I.Y.; Kholdeeva, O.A. Nucleophilic versus Electrophilic Activation of Hydrogen Peroxide over Zr-Based Metal–Organic Frameworks. Inorg. Chem. 2020, 59, 10634–10649. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.B.; Deming, P.H.; Williams, P.H. Reactions of Hydrogen Peroxide. VII. Alkali-Catalyzed Epoxidation and Oxidation Using a Nitrile as Co-reactant. J. Org. Chem. 1961, 26, 659–663. [Google Scholar] [CrossRef]
- Huang, K.; Yu, S.; Li, X.; Cai, Z. One-pot synthesis of bimetal MOFs as highly efficient catalysts for selective oxidation of styrene. J. Chem. Sci. 2020, 132, 139. [Google Scholar] [CrossRef]
- Wang, F.; Meng, X.-G.; Wu, Y.-Y.; Huang, H.; Lv, J.; Yu, W.-W. A Highly Efficient Heterogeneous Catalyst of Bimetal-Organic Frameworks for the Epoxidation of Olefin with H2O2. Molecules 2020, 25, 2389. [Google Scholar] [CrossRef]
- Santibáñez, L.; Escalona, N.; Torres, J.; Kremer, C.; Cancino, P.; Spodine, E. CuII- and CoII-based MOFs: {[La2Cu3(µ-H2O)(ODA)6(H2O)3]·3H2O}n and {[La2Co3(ODA)6(H2O)6]·12H2O}n. The Relevance of Physicochemical Properties on the Catalytic Aerobic Oxidation of Cyclohexene. Catalysts 2020, 10, 589. [Google Scholar] [CrossRef]
- Tuci, G.; Giambastiani, G.; Kwon, S.; Stair, P.C.; Snurr, R.Q.; Rossin, A. Chiral Co(II) Metal–Organic Framework in the Heterogeneous Catalytic Oxidation of Alkenes under Aerobic and Anaerobic Conditions. ACS Catal. 2014, 4, 1032–1039. [Google Scholar] [CrossRef]
- Otake, K.-I.; Ahn, S.; Knapp, J.; Hupp, J.T.; Notestein, J.M.; Farha, O.K. Vapor-Phase Cyclohexene Epoxidation by Single-Ion Fe(III) Sites in Metal–Organic Frameworks. Inorg. Chem. 2021, 60, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Thornburg, N.; Li, Z.; Wang, T.C.; Gallington, L.; Chapman, K.W.; Notestein, J.M.; Hupp, J.T.; Farha, O.K. Stable Metal–Organic Framework-Supported Niobium Catalysts. Inorg. Chem. 2016, 55, 11954–11961. [Google Scholar] [CrossRef]
- Thornburg, N.E.; Nauert, S.L.; Thompson, A.B.; Notestein, J.M. Synthesis−Structure–Function Relationships of Silica-Supported Niobium(V) Catalysts for Alkene Epoxidation with H2O2. ACS Catal. 2016, 6, 6124–6134. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhang, Z.; Li, X.; Tian, H.; Yan, T.; Zhang, X.; Liu, S.; Sun, X.; Xu, L.; et al. One-Step Template-Free Fabrication of Ultrathin Mixed-Valence Polyoxovanadate-Incorporated Metal–Organic Framework Nanosheets for Highly Efficient Selective Oxidation Catalysis in Air. ACS Appl. Mater. Interfaces 2019, 11, 12786–12796. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.; Chu, H.; Zhang, W.; Shen, Y.; Chen, W.; Hu, Y.; Liu, W.; Gao, C.; Guo, S.; Xiao, G.; et al. Multicomponent metal–organic framework derivatives for optimizing the selective catalytic performance of styrene epoxidation reaction. Nanoscale 2018, 10, 8772–8778. [Google Scholar] [CrossRef] [PubMed]
- Saedi, Z.; Safarifard, V.; Morsali, A. Dative and covalent-dative postsynthetic modification of a two-fold interpenetration pillared-layer MOF for heterogeneous catalysis: A comparison of catalytic activities and reusability. Microporous Mesoporous Mater. 2016, 229, 51–58. [Google Scholar] [CrossRef]
- Berijani, K.; Morsali, A.; Hupp, J.T. An effective strategy for creating asymmetric MOFs for chirality induction: A chiral Zr-based MOF for enantioselective epoxidation. Catal. Sci. Technol. 2019, 9, 3388–3397. [Google Scholar] [CrossRef]
- Noh, H.; Cui, Y.; Peters, A.W.; Pahls, D.; Ortuño, M.A.; Vermeulen, N.A.; Cramer, C.J.; Gagliardi, L.; Hupp, J.T.; Farha, O.K. An Exceptionally Stable Metal–Organic Framework Supported Molybdenum(VI) Oxide Catalyst for Cyclohexene Epoxidation. J. Am. Chem. Soc. 2016, 138, 14720–14726. [Google Scholar] [CrossRef]
- Kardanpour, R.; Tangestaninejad, S.; Mirkhani, V.; Moghadam, M.; Mohammadpoor-Baltork, I.; Zadehahmadi, F. Efficient alkene epoxidation catalyzed by molybdenyl acetylacetonate supported on aminated UiO-66 metal−organic framework. J. Solid State Chem. 2015, 226, 262–272. [Google Scholar] [CrossRef]
- Tang, J.; Dong, W.; Wang, G.; Yao, Y.; Cai, L.; Liu, Y.; Zhao, X.; Xu, J.; Tan, L. Efficient molybdenum(vi) modified Zr-MOF catalysts for epoxidation of olefins. RSC Adv. 2014, 4, 42977–42982. [Google Scholar] [CrossRef]
- Liu, T.-F.; Vermeulen, N.A.; Howarth, A.J.; Li, P.; Sarjeant, A.A.; Hupp, J.T.; Farha, O.K. Adding to the Arsenal of Zirconium-Based Metal–Organic Frameworks: The Topology as a Platform for Solvent-Assisted Metal Incorporation. Eur. J. Inorg. Chem. 2016, 27, 4349. [Google Scholar] [CrossRef]
- Pereira, C.; Biernacki, K.; Rebelo, S.L.; Magalhães, A.L.; Carvalho, A.P.; Pires, J.; Freire, C. Designing heterogeneous oxovanadium and copper acetylacetonate catalysts: Effect of covalent immobilisation in epoxidation and aziridination reactions. J. Mol. Catal. A Chem. 2009, 312, 53–64. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Ding, H.; Lu, H.; Liu, J.; Huo, Q.; Guan, J.; Kan, Q. Oxovanadium(iv) and iron(iii) salen complexes immobilized on amino-functionalized graphene oxide for the aerobic epoxidation of styrene. New J. Chem. 2013, 37, 4220–4229. [Google Scholar] [CrossRef]
- Ben Zid, T.; Khedher, I.; Ghorbel, A. Chiral vanadyl salen catalyst immobilized on mesoporous silica as support for asymmetric oxidation of sulfides to sulfoxides. React. Kinet. Mech. Catal. 2010, 100, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Zamanifar, E.; Farzaneh, F. Immobilized vanadium amino acid Schiff base complex on Al-MCM-41 as catalyst for the epoxidation of allyl alcohols. React. Kinet. Mech. Catal. 2011, 104, 197–209. [Google Scholar] [CrossRef]
- Pourkhosravani, M.; Dehghanpour, S.; Farzaneh, F.; Sohrabi, S. Designing new catalytic nanoreactors for the regioselective epoxidation of geraniol by the post-synthetic immobilization of oxovanadium(IV) complexes on a ZrIV-based metal–organic framework. React. Kinet. Mech. Catal. 2017, 122, 961–981. [Google Scholar] [CrossRef]
- Tan, C.; Han, X.; Li, Z.; Liu, Y.; Cui, Y. Controlled Exchange of Achiral Linkers with Chiral Linkers in Zr-Based UiO-68 Metal–Organic Framework. J. Am. Chem. Soc. 2018, 140, 16229–16236. [Google Scholar] [CrossRef]
- Huang, K.; Guo, L.L.; Wu, D.F. Synthesis of Metal Salen@MOFs and Their Catalytic Performance for Styrene Oxidation. Ind. Eng. Chem. Res. 2019, 58, 4744–4754. [Google Scholar] [CrossRef]
- Xia, Q.; Li, Z.; Tan, C.; Liu, Y.; Gong, W.; Cui, Y. Multivariate Metal–Organic Frameworks as Multifunctional Heterogeneous Asymmetric Catalysts for Sequential Reactions. J. Am. Chem. Soc. 2017, 139, 8259–8266. [Google Scholar] [CrossRef]
- Berijani, K.; Morsali, A. Construction of an Asymmetric Porphyrinic Zirconium Metal–Organic Framework through Ionic Postchiral Modification. Inorg. Chem. 2021, 60, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Martínez, H.; Cáceres, M.F.; Martínez, F.; Páez-Mozo, E.A.; Valange, S.; Castellanos, N.J.; Molina, D.; Barrault, J.; Arzoumanian, H. Photo-epoxidation of cyclohexene, cyclooctene and 1-octene with molecular oxygen catalyzed by dichloro dioxo-(4,4′-dicarboxylato-2,2′-bipyridine) molybdenum(VI) grafted on mesoporous TiO2. J. Mol. Catal. A Chem. 2016, 423, 248–255. [Google Scholar] [CrossRef]
- Portillo, A.S.; Navalón, S.; Cirujano, F.G.; I Xamena, F.X.L.; Alvaro, M.; Garcia, H. MIL-101 as Reusable Solid Catalyst for Autoxidation of Benzylic Hydrocarbons in the Absence of Additional Oxidizing Reagents. ACS Catal. 2015, 5, 3216–3224. [Google Scholar] [CrossRef]
- Kou, J.; Sun, L.-B. Fabrication of Metal–Organic Frameworks inside Silica Nanopores with Significantly Enhanced Hydrostability and Catalytic Activity. ACS Appl. Mater. Interfaces 2018, 10, 12051–12059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, X.; Wang, Y.; Luan, Y.; Li, X.; Du, X. Growth of Cu-BTC MOFs on dendrimer-like porous silica nanospheres for the catalytic aerobic epoxidation of olefins. New J. Chem. 2020, 44, 14350–14357. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Tang, H.; Ramella, D.; Luan, Y. Modification of Cu2+ into Zr-based metal–organic framework (MOF) with carboxylic units as an efficient heterogeneous catalyst for aerobic epoxidation of olefins. Mol. Catal. 2018, 456, 57–64. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Guo, S.; Zeng, Y.; Ding, M. MOFs-induced high-amphiphilicity in hierarchical 3D reduced graphene oxide-based hydrogel. Appl. Surf. Sci. 2021, 540, 148303. [Google Scholar] [CrossRef]
- Canioni, R.; Roch-Marchal, C.; Sécheresse, F.; Horcajada, P.; Serre, C.; Hardi-Dan, M.; Férey, G.; Grenèche, J.-M.; Lefebvre, F.; Chang, J.-S.; et al. Stable polyoxometalate insertion within the mesoporous metal organic framework MIL-100(Fe). J. Mater. Chem. 2011, 21, 1226–1233. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yang, G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef]
- Song, X.; Hu, D.; Yang, X.; Zhang, H.; Zhang, W.; Li, J.; Jia, M.; Yu, J. Polyoxomolybdic Cobalt Encapsulated within Zr-Based Metal–Organic Frameworks as Efficient Heterogeneous Catalysts for Olefins Epoxidation. ACS Sustain. Chem. Eng. 2019, 7, 3624–3631. [Google Scholar] [CrossRef]
- Villabrille, P.; Romanelli, G.; Gassa, L.; Vazquez, P.; Caceres, C. Synthesis and characterization of Fe- and Cu-doped molybdovanadophosphoric acids and their application in catalytic oxidation. Appl. Catal. A Gen. 2007, 324, 69–76. [Google Scholar] [CrossRef]
- Jin, M.; Niu, Q.; Liu, G.; Lv, Z.; Si, C.; Guo, H. Encapsulation of ionic liquids into POMs-based metal–organic frameworks: Screening of POMs-ILs@MOF catalysts for efficient cycloolefins epoxidation. J. Mater. Sci. 2020, 55, 8199–8210. [Google Scholar] [CrossRef]
- Ke, F.; Guo, F.; Yu, J.; Yang, Y.; He, Y.; Chang, L.; Wan, X. Highly Site-Selective Epoxidation of Polyene Catalyzed by Metal–Organic Frameworks Assisted by Polyoxometalate. J. Inorg. Organomet. Polym. Mater. 2017, 27, 843–849. [Google Scholar] [CrossRef]
- Li, N.; Mu, B.; Lv, L.; Huang, R. Assembly of new polyoxometalate–templated metal–organic frameworks based on flexible ligands. J. Solid State Chem. 2015, 226, 88–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.-X.; Liu, L.; Han, Z.-B. Palladium nanoparticles supported on UiO-66-NH2 as heterogeneous catalyst for epoxidation of styrene. Inorg. Chem. Commun. 2019, 100, 51–55. [Google Scholar] [CrossRef]
- Limvorapitux, R.; Chou, L.-Y.; Young, A.P.; Tsung, C.-K.; Nguyen, S.T. Coupling Molecular and Nanoparticle Catalysts on Single Metal–Organic Framework Microcrystals for the Tandem Reaction of H2O2 Generation and Selective Alkene Oxidation. ACS Catal. 2017, 7, 6691–6698. [Google Scholar] [CrossRef]
- Jin, T.; Yang, Q.; Meng, C.; Xu, J.; Liu, H.; Hu, J.; Ling, H. Promoting desulfurization capacity and separation efficiency simultaneously by the novel magnetic Fe3O4@PAA@MOF-199. RSC Adv. 2014, 4, 41902–41909. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Tan, L.; Luan, Y.; Yang, M. Superparamagnetic Core-Shell Metal-Organic Framework Fe3O4/Cu3(btc)2Microspheres and Their Catalytic Activity in the Aerobic Oxidation of Alcohols and Olefins. Eur. J. Inorg. Chem. 2016, 2016, 4906–4912. [Google Scholar] [CrossRef]
- Hou, J.; Luan, Y.; Yu, J.; Qi, Y.; Wang, G.; Lu, Y. Fabrication of hierarchical composite microspheres of copper-doped Fe3O4@P4VP@ZIF-8 and their application in aerobic oxidation. New J. Chem. 2016, 40, 10127–10135. [Google Scholar] [CrossRef]
- Qi, Y.; Luan, Y.; Yu, J.; Peng, X.; Wang, G. Nanoscaled Copper Metal-Organic Framework (MOF) Based on Carboxylate Ligands as an Efficient Heterogeneous Catalyst for Aerobic Epoxidation of Olefins and Oxidation of Benzylic and Allylic Alcohols. Chem. A Eur. J. 2015, 21, 1589–1597. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, S.; Zou, L.; Drake, H.; Zhang, Y.; Qin, J.; Alsalme, A.; Zhou, H.C. One-Step Synthesis of Hybrid Core–Shell Metal–Organic Frameworks. Angew. Chem. 2018, 130, 3991–3996. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2013, 114, 1709–1742. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R. Mechanism of Cyclic Carbonate Synthesis from Epoxides and CO2. Angew. Chem. Int. Ed. 2009, 48, 2946–2948. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Xu, S.; Zhang, Q.; Liu, T.; Wei, B.; Zhao, Y.; Meng, L.; Li, J. Ionic Liquid/Quaternary Ammonium Salt Integrated Heterogeneous Catalytic System for the Efficient Coupling of Carbon Dioxide with Epoxides. Ind. Eng. Chem. Res. 2018, 57, 15319–15328. [Google Scholar] [CrossRef]
- Shi, X.-L.; Chen, Y.; Duan, P.; Zhang, W.; Hu, Q. Conversion of CO2 into Organic Carbonates over a Fiber-Supported Ionic Liquid Catalyst in Impellers of the Agitation System. ACS Sustain. Chem. Eng. 2018, 6, 7119–7127. [Google Scholar] [CrossRef]
- Ziaee, M.A.; Tang, Y.; Zhong, H.; Tian, D.; Wang, R. Urea-Functionalized Imidazolium-Based Ionic Polymer for Chemical Conversion of CO2 into Organic Carbonates. ACS Sustain. Chem. Eng. 2018, 7, 2380–2387. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, B.; Zhang, H.; Zhao, Y.; Chen, Y.; Ma, Z.; Ji, G.; Gao, X.; Han, B.; Liu, Z. Metalated Mesoporous Poly(triphenylphosphine) with Azo Functionality: Efficient Catalysts for CO2 Conversion. ACS Catal. 2016, 6, 1268–1273. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Perman, J.A.; Nguyen, N.T.-K.; Ma, S. Flexibility Matters: Cooperative Active Sites in Covalent Organic Framework and Threaded Ionic Polymer. J. Am. Chem. Soc. 2016, 138, 15790–15796. [Google Scholar] [CrossRef]
- Beyzavi, M.H.; Stephenson, C.J.; Liu, Y.; Karagiaridi, O.; Hupp, J.T.; Farha, O.K. Metal-organic framework-based catalysts: Chemical fixation of CO2 with epoxides leading to cyclic organic carbonates. Front. Energy Res. 2015, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Bon, V.; Senkovskyy, V.; Senkovska, I.; Kaskel, S. Zr(IV) and Hf(IV) based metal–organic frameworks with reo-topology. Chem. Commun. 2012, 48, 8407–8409. [Google Scholar] [CrossRef] [Green Version]
- Beyzavi, M.H.; Klet, R.C.; Tussupbayev, S.; Borycz, J.; Vermeulen, N.A.; Cramer, C.J.; Stoddart, J.F.; Hupp, J.T.; Farha, O.K. A Hafnium-Based Metal–Organic Framework as an Efficient and Multifunctional Catalyst for Facile CO2 Fixation and Regioselective and Enantioretentive Epoxide Activation. J. Am. Chem. Soc. 2014, 136, 15861–15864. [Google Scholar] [CrossRef]
- Tran, Y.B.N.; Nguyen, P.T.K.; Luong, Q.T.; Nguyen, K.D. Series of M-MOF-184 (M = Mg, Co, Ni, Zn, Cu, Fe) Metal-Organic Frameworks for Catalysis Cycloaddition of CO2. Inorg. Chem. 2020, 59, 16747–16759. [Google Scholar] [CrossRef]
- Agarwal, R.A.; Gupta, A.K.; De, D. Flexible Zn-MOF Exhibiting Selective CO2 Adsorption and Efficient Lewis Acidic Catalytic Activity. Cryst. Growth Des. 2019, 19, 2010–2018. [Google Scholar] [CrossRef]
- Hayashi, H.; Côté, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Zeolite A imidazolate frameworks. Nat. Mater. 2007, 6, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Chen, Y.; Wang, N.; Hu, Z.; Jiang, J.; Caro, J. A highly permeable and selective zeolitic imidazolate framework ZIF-95 membrane for H2/CO2 separation. Chem. Commun. 2012, 48, 10981–10983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhin, K.M.; Tharun, J.; Roshan, K.R.; Kim, D.-W.; Chung, Y.; Park, D.-W. Catalytic performance of zeolitic imidazolate framework ZIF-95 for the solventless synthesis of cyclic carbonates from CO2 and epoxides. J. CO2 Util. 2017, 17, 112–118. [Google Scholar] [CrossRef]
- Liang, J.; Chen, R.-P.; Wang, X.-Y.; Liu, T.-T.; Wang, X.-S.; Huang, Y.-B.; Cao, R. Postsynthetic ionization of an imidazole-containing metal–organic framework for the cycloaddition of carbon dioxide and epoxides. Chem. Sci. 2017, 8, 1570–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.-G.; Yao, B.-J.; Jiang, W.-L.; Li, J.-T.; Fu, Q.-J.; Li, Y.-A.; Liu, Z.-H.; Ma, J.-P.; Dong, Y.-B. Bifunctional Imidazolium-Based Ionic Liquid Decorated UiO-67 Type MOF for Selective CO2 Adsorption and Catalytic Property for CO2 Cycloaddition with Epoxides. Inorg. Chem. 2017, 56, 2337–2344. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Rachuri, Y.; Pillai, R.S.; Gu, Y.; Choe, Y.; Park, D. Ionic-Liquid-Functionalized UiO-66 Framework: An Experimental and Theoretical Study on the Cycloaddition of CO2 and Epoxides. ChemSusChem 2019, 12, 1033–1042. [Google Scholar] [CrossRef]
- Ding, M.; Jiang, H.-L. Incorporation of Imidazolium-Based Poly(ionic liquid)s into a Metal–Organic Framework for CO2 Capture and Conversion. ACS Catal. 2018, 8, 3194–3201. [Google Scholar] [CrossRef]
- Pander, M.; Janeta, M.; Bury, W. Quest for an Efficient 2-in-1 MOF-Based Catalytic System for Cycloaddition of CO2 to Epoxides under Mild Conditions. ACS Appl. Mater. Interfaces 2021, 13, 8344–8352. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Usov, P.M.; Xu, W.; Celis-Salazar, P.J.; Lin, S.; Kessinger, M.C.; Landaverde-Alvarado, C.; Cai, M.; May, A.M.; Slebodnick, C.; et al. A New Class of Metal-Cyclam-Based Zirconium Metal–Organic Frameworks for CO2 Adsorption and Chemical Fixation. J. Am. Chem. Soc. 2018, 140, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Vitillo, J.G.; Savonnet, M.; Ricchiardi, G.; Bordiga, S. Tailoring Metal-Organic Frameworks for CO2 Capture: The Amino Effect. ChemSusChem 2011, 4, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, A.; Bell, R.G.; Mellot-Draznieks, C. Functionalized MOFs for Enhanced CO2 Capture. Cryst. Growth Des. 2010, 10, 2839–2841. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Maru, M.S.; Somani, R.S.; Bajaj, H.C.; Neogi, S. Unprecedented NH2-MIL-101(Al)/n-Bu4NBr system as solvent-free heterogeneous catalyst for efficient synthesis of cyclic carbonates via CO2 cycloaddition. Dalton Trans. 2018, 47, 418–428. [Google Scholar] [CrossRef]
- Zhu, M.; Carreon, M.A. Porous crystals as active catalysts for the synthesis of cyclic carbonates. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Cai, T.; Liu, J.; Cao, H.; Cui, C. Synthesis of bio-based cyclic carbonate from vegetable oil methyl ester by CO2 fixation with acid-base pair MOFs. Ind. Crop. Prod. 2020, 145, 112155. [Google Scholar] [CrossRef]
- Mercuri, G.; Moroni, M.; Domasevitch, K.V.; Di Nicola, C.; Campitelli, P.; Pettinari, C.; Giambastiani, G.; Galli, S.; Rossin, A. Carbon Dioxide Capture and Utilization with Isomeric Forms of Bis(amino)-Tagged Zinc Bipyrazolate Metal–Organic Frameworks. Chem. A Eur. J. 2021, 27, 4746–4754. [Google Scholar] [CrossRef]
- Norouzi, F.; Khavasi, H.R. Diversity-Oriented Metal Decoration on UiO-Type Metal-Organic Frameworks: An Efficient Approach to Increase CO2 Uptake and Catalytic Conversion to Cyclic Carbonates. ACS Omega 2019, 4, 19037–19045. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Yue, C.; Fan, Y.; Qi, C.; Jiang, H. Highly Stable Chiral Zirconium–Metallosalen Frameworks for CO2 Conversion and Asymmetric C–H Azidation. ACS Appl. Mater. Interfaces 2018, 10, 36047–36057. [Google Scholar] [CrossRef]









| MOF | Substrate | Reaction Data T (°C) P (atm) Time (h) | Oxidant/Cocatalyst/ Solvent a | Conversion % | Epoxide Selectivity% | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| UiO-66-SI/VO(acac) | Geraniol | 40 | 1 | 1 | TBHP/-/CH2Cl2 | 100 | 100 | [67] |
| UiO-66-N/VO(acac)2 | Geraniol | 40 | 1 | 2 | TBHP/-/CH2Cl2 | 100 | 100 | [67] |
| UiO-66-sal-MoD | cis-Cyclooctene | 80 | 24 | 1 | TBHP/-/CH3CN | 99 | 99 | [61] |
| PCN-224-Mn(tart) | Styrene | 60 | 1 | 4 | O2/IBA/CH3CN | 100 | 89 | [71] |
| trans-Stilbene | 60 | 1 | 4 | O2/IBA/CH3CN | 80 | 100 | [71] | |
| 1-Phenyl-1-cyclohexene | 60 | 1 | 4 | O2/IBA/CH3CN | 75 | 100 | [71] | |
| 1-Octene | 60 | 1 | 4 | O2/IBA/CH3CN | 70 | 100 | [71] | |
| UiO-67-Mo(CO)3 | Cyclooctene | 55 | 3 | 1 | TBHP/-/toluene | 100 | 99 | [33] |
| UiO-66-Mo(CO)3 | Cyclooctene | 55 | 3 | 1 | TBHP/-/toluene | 92 | 99 | [33] |
| [C-NU-1000-Mo] | Styrene | 120 | 0.03 | 5 | H2O2/-/CH2CH2Cl2 | 100 | 86 | [58] |
| 1-Octene | 120 | 0.03 | 8 | H2O2/-/CH2CH2Cl2 | 72 | 100 | [58] | |
| UiO-66-NH2-SA-Mo | Cyclooctene | 83 | 0.75 | 1 | TBHP/-/CH2CH2Cl2 | 97 | 100 | [60] |
| Cyclohexene | 83 | 1.5 | 1 | TBHP/-/CH2CH2Cl2 | 93 | 100 | [60] | |
| Styrene | 83 | 5 | 1 | TBHP/-/CH2CH2Cl2 | 87 | 92 | [60] | |
| 1-Octene | 83 | 8 | 1 | TBHP/-/CH2CH2Cl2 | 78 | 100 | [60] | |
| 1-Decene | 83 | 10 | 1 | TBHP/-/CH2CH2Cl2 | 79 | 100 | [60] | |
| UiO-66-NH2-TC-Mo | Cyclooctene | 83 | 1 | 1 | TBHP/-/CH2CH2Cl2 | 94 | 100 | [60] |
| Cyclohexene | 83 | 2 | 1 | TBHP/-/CH2CH2Cl2 | 90 | 100 | [60] | |
| Styrene | 83 | 5 | 1 | TBHP/-/CH2CH2Cl2 | 86 | 90 | [60] | |
| 1-Octene | 83 | 8 | 1 | TBHP/-/CH2CH2Cl2 | 75 | 100 | [60] | |
| 1-Decene | 83 | 10.5 | 1 | TBHP/-/CH2CH2Cl2 | 75 | 100 | [60] | |
| [Zn4O(L5Cu,Fe)3] | 2,2-Dimethyl-2H-chromene | −20 | 1 | 36 | MesPhIO/-/CHCl3 | 94 | 87 ee | [70] |
| [Zn4O(L5Cu,Mn,Co)3] | 3-Chloropropene | 0 | 1 | 10 | sPhIO/-/CHCl3 | 92 | - | [70] |
| Styrene | 0 | 1 | 24 | sPhIO/-/CH2Cl2 | 63 | - | [70] | |
| UiO-66-PC-MoD | cis-Cyclooctene | 80 | 24 | 1 | TBHP/-/CH3CN | 90.7 | 99 | [61] |
| Mo-SIM | Cyclohexene | 60 | 1 | 7 | TBHP/-/toluene | 93 | 99 | [59] |
| (R)-UiO-68-Mn | 2,2-Dimethyl-2H-chromene | 0 | 1 | 10 | sPhIO/-/CH2Cl2 | 91 | 88 ee | [68] |
| UiO-66-PI-MoD | cis-Cyclooctene | 80 | 24 | 1 | TBHP/-/CH3CN | 77.5 | 99 | [61] |
| (R)-UiO-68-Fe | 2,2-Dimethyl-2H-chromene | −20 | 1 | 36 | MesPhIO/-/CHCl3 | 84 | 86 ee | [68] |
| Cusalen@NH2-MIL-101(Cr) | Styrene | 80 | 1 | 6 | TBHP/-/CH3CN | 98.78 | 89.58 | [69] |
| [Zn4O(L5Cu,Mn)3] | 2,2-Dimethyl-2H-chromene | −20 | 1 | 36 | MesPhIO/-/CH2Cl2 | 86 | 86 ee | [70] |
| PCN-224-Mn(tart) | Styrene | 60 °C | 1 | 4 | O2/IBA/CH3CN | 100 | 89 | [71] |
| trans-Stilbene | 60 °C | 1 | 4 | O2/IBA/CH3CN | 80 | 100 | [71] | |
| 1-Phenyl-1-cyclohexene | 60 °C | 1 | 4 | O2/IBA/CH3CN | 75 | 100 | [71] | |
| 1-Octene | 60 °C | 1 | 4 | O2/IBA/CH3CN | 70 | 100 | [71] | |
| TMU-16-NH2 | Cyclohexene | 60 | 1 | 40 | TBHP/-/CHCl3 | 66 | 74 | [57] |
| Styrene | 60 | 1 | 51 | TBHP/-/CHCl3 | 88 | 98 | [57] | |
| Cyclooctene | 60 | 1 | 24 | TBHP/-/CHCl3 | 83 | 83 | [57] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tombesi, A.; Pettinari, C. Metal Organic Frameworks as Heterogeneous Catalysts in Olefin Epoxidation and Carbon Dioxide Cycloaddition. Inorganics 2021, 9, 81. https://doi.org/10.3390/inorganics9110081
Tombesi A, Pettinari C. Metal Organic Frameworks as Heterogeneous Catalysts in Olefin Epoxidation and Carbon Dioxide Cycloaddition. Inorganics. 2021; 9(11):81. https://doi.org/10.3390/inorganics9110081
Chicago/Turabian StyleTombesi, Alessia, and Claudio Pettinari. 2021. "Metal Organic Frameworks as Heterogeneous Catalysts in Olefin Epoxidation and Carbon Dioxide Cycloaddition" Inorganics 9, no. 11: 81. https://doi.org/10.3390/inorganics9110081
APA StyleTombesi, A., & Pettinari, C. (2021). Metal Organic Frameworks as Heterogeneous Catalysts in Olefin Epoxidation and Carbon Dioxide Cycloaddition. Inorganics, 9(11), 81. https://doi.org/10.3390/inorganics9110081