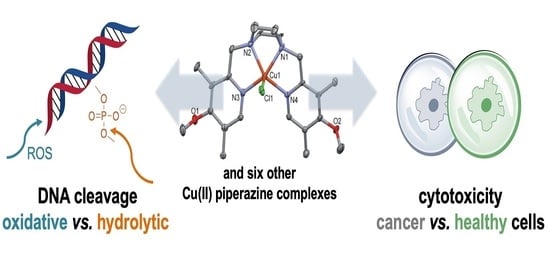
Copper(II) Complexes with Tetradentate Piperazine-Based Ligands: DNA Cleavage and Cytotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses
2.2. Characterization of the Complexes
2.2.1. Crystal Structures of Complexes 4-Cl and 5-Cl
2.2.2. Spectroscopic and Molar Conductivity Characterization
2.2.3. Solution Study and Stability of Complexes
2.3. DNA Cleavage Studies
2.3.1. Concentration Dependence
2.3.2. Kinetics of DNA Cleavage
2.4. BNPP Assay
2.5. Reactive Oxygen Species (ROS)
2.6. Cytotoxicity Studies
3. Materials and Methods
3.1. General
3.2. Syntheses
3.2.1. 1,4-Bis[(6-methyl)-(2-pyridinyl)methyl]piperazine (L2·¼H2O)
3.2.2. 1,4-Bis[(2-quinolyl)methyl]piperazine (L3·¼H2O)
3.2.3. 1,4-Bis[(3,4-dimethoxy-2-pyridinyl)methyl)]piperazine (L4·¼H2O)
3.2.4. 1,4-Bis[(3,5-dimethyl-4-methoxy-2-pyridinyl)methyl)]piperazine (L5):
3.2.5. Synthesis of Cu(II) Complexes
3.2.6. [Cu(L2)ClO4]ClO4 (2-ClO4)
3.2.7. [Cu(L3)ClO4]ClO4 (3-ClO4)
3.2.8. [Cu(L4)Cl]ClO4·½H2O (4-Cl)
3.2.9. [Cu(L5)Cl]PF6 (5-Cl)
3.2.10. [Cu(L1)Cl]PF6 (1-Cl)
3.3. DNA Cleavage
3.3.1. Kinetics of DNA Cleavage
3.3.2. ROS Scavenger Study
3.3.3. Cleavage of BNPP
3.3.4. Cell Culture and Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schroeder, G.K.; Lad, C.; Wyman, P.; Williams, N.H.; Wolfenden, R. The time required for water attack at the phosphorus atom of simple phosphodiesters and of DNA. Proc. Natl. Acad. Sci. USA 2006, 103, 4052–4255. [Google Scholar] [CrossRef] [Green Version]
- Cowan, J.A. Role of Metal Ions in Promoting DNA Binding and Cleavage by Restriction Endonucleases. In Restriction Endonucleases; Pingoud, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 14, pp. 339–360. [Google Scholar]
- Doniz Kettenmann, S.; Louka, F.R.; Marine, E.; Fischer, R.C.; Mautner, F.A.; Kulak, N.; Massoud, S.S. Efficient Artificial Nucleases for Mediating DNA Cleavage Based on Tuning the Steric Effect in the Pyridyl Derivatives of Tripod Tetraamine-Cobalt(II) Complexes. Eur. J. Inorg. Chem. 2018, 2018, 2322–2338. [Google Scholar] [CrossRef]
- Massoud, S.S.; Perkins, R.S.; Louka, F.R.; Xu, W.; le Roux, A.; Dutercq, Q.; Fischer, R.C.; Mautner, F.A.; Handa, M.; Hiraoka, Y.; et al. Efficient hydrolytic cleavage of plasmid DNA by chloro-cobalt(II) complexes based on sterically hindered pyridyl tripod tetraamine ligands: Synthesis, crystal structure and DNA cleavage. Dalton Trans. 2014, 43, 10086–10103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massoud, S.S.; Ledet, C.C.; Junk, T.; Bosch, S.; Comba, P.; Herchel, R.; Hošek, J.; Trávníček, Z.; Fischer, R.C.; Mautner, F.A. Dinuclear metal(II)-acetato complexes based on bicompartmental 4-chlorophenolate: Syntheses, structures, magnetic properties, DNA interactions and phosphodiester hydrolysis. Dalton Trans. 2016, 45, 12933–12950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massoud, S.S.; Perkins, R.S.; Knierim, K.D.; Comiskey, S.P.; Otero, K.H.; Michel, C.L.; Juneau, W.M.; Albering, J.H.; Mautner, F.A.; Xu, W. Effect of the chelate ring size on the cleavage activity of DNA by copper(II) complexes containing pyridyl groups. Inorg. Chim. Acta 2013, 339, 177–184. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Xu, W.; Perkins, R.; Vicente, R.; Albering, J.H.; Mautner, F.A. DNA Cleavage by Structurally Characterized Dinuclear Copper(II) Complexes Based on Triazine. Eur. J. Inorg. Chem. 2011, 2011, 3469–3479. [Google Scholar] [CrossRef]
- Sureshbabu, P.; Sudarga Tjakraatmadja, A.A.J.; Hanmandlu, C.; Elavarasan, K.; Kulak, N.; Sabiah, S. Mononuclear Cu(II) and Zn(II) complexes with a simple diamine ligand: Synthesis, structure, phosphodiester binding and DNA cleavage studies. RSC Adv. 2015, 5, 22405–22418. [Google Scholar] [CrossRef]
- Hormann, J.; van der Meer, M.; Sarkar, B.; Kulak, N. From Cyclen to 12-Crown-4 Copper(II) Complexes: Exchange of Donor Atoms Improves DNA Cleavage Activity. Eur. J. Inorg. Chem. 2015, 2015, 4722–4730. [Google Scholar] [CrossRef]
- Wende, C.; Lüdtke, C.; Kulak, N. Copper Complexes of N-Donor Ligands as Artificial Nucleases. Eur. J. Inorg. Chem. 2014, 2014, 2597–2612. [Google Scholar] [CrossRef]
- Sudarga Tjakraatmadja, A.A.J.; Lüdtke, C.; Kulak, N. Tuning the DNA binding and cleavage of bpa Cu(II) complexes by ether tethers with hydroxyl and methoxy groups. Inorg. Chim. Acta 2016, 452, 159–169. [Google Scholar] [CrossRef]
- Soler, M.; Figueras, E.; Serrano-Plana, J.; González-Bártulos, M.; Massaguer, A.; Company, A.; Martínez, M.A.; Malina, J.; Brabec, V.; Feliu, L.; et al. Design, Preparation, and Characterization of Zn and Cu Metallopeptides Based On Tetradentate Aminopyridine Ligands Showing Enhanced DNA Cleavage Activity. Inorg. Chem. 2015, 54, 10542–10558. [Google Scholar] [CrossRef] [PubMed]
- Mandegani, Z.; Asadi, Z.; Asadi, M.; Karbalaei-Heidari, H.R.; Rastegari, B. Synthesis, characterization, DNA binding, cleavage activity, cytotoxicity and molecular docking of new nano water-soluble [M(5-CH2PPh3-3,4-salpyr)](ClO4)2 (M = Ni, Zn) complexes. Dalton Trans. 2016, 45, 6592–6611. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.M.G.; Lima, L.M.P.; Gama, S.; Mendes, F.; Orio, M.; Bento, I.; Paulo, A.; Delgado, R.; Iranzo, O. Copper(II) Complexes of Phenanthroline and Histidine Containing Ligands: Synthesis, Characterization and Evaluation of their DNA Cleavage and Cytotoxic Activity. Inorg. Chem. 2016, 55, 11801–11814. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, L.; Gu, W.; Liu, X.; Tian, J.; Yan, S.-P. Efficient double-strand cleavage of DNA mediated by Zn(II)-based artificial nucleases. Dalton Trans. 2011, 40, 5617–5624. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Tian, J.-L.; Gu, W.; Liu, X.; Yan, S.-P. A novel 1,2,4-triazole-based copper(II) complex: Synthesis, characterization, magnetic property and nuclease activity. J. Inorg. Biochem. 2010, 104, 171–179. [Google Scholar] [CrossRef]
- Wang, J.T.; Xia, Q.; Zheng, X.-H.; Chen, H.-Y.; Chao, H.; Mao, Z.-W.; Ji, L.N. An effective approach to artificial nucleases using copper(II) complexes bearing nucleobases. Dalton Trans. 2010, 39, 2128–2136. [Google Scholar] [CrossRef]
- Sreedhara, A.; Freed, J.D.; Cowan, J.A. Efficient Inorganic Deoxyribonucleases. Greater than 50-Million-Fold Rate Enhancement in Enzyme-Like DNA Cleavage. J. Am. Chem. Soc. 2000, 122, 8814–8824. [Google Scholar] [CrossRef]
- Parveen, S.; Cowan, J.A.; Zhen Yu, Z.; Arjmand, F. Enantiomeric copper based anticancer agents promoting sequence-selective cleavage of G-quadruplex telomeric DNA and non-random cleavage of plasmid DNA. Metallomics 2020, 12, 988–999. [Google Scholar] [CrossRef]
- Majumder, S.; Pasayat, S.; Panda, A.K.; Dash, S.P.; Roy, S.; Biswas, A.; Varma, M.E.; Joshi, B.N.; Garribba, E.; Kausar, C.; et al. Monomeric and Dimeric Oxidomolybdenum(V and VI) Complexes, Cytotoxicity, and DNA Interaction Studies: Molybdenum Assisted C=N Bond Cleavage of Salophen Ligands. Inorg. Chem. 2017, 56, 11190–11210. [Google Scholar] [CrossRef]
- Yu, Z.; Cowan, J.A. Metal Complexes Promoting Catalytic Cleavage of Nucleic Acids—Biochemical Tools and Therapeutics. Curr. Opin Chem. Biol. 2018, 43, 37–42. [Google Scholar] [CrossRef]
- Peng, B.; Gao, Z.; Li, X.; Li, T.; Chen, G.; Zhou, M.; Zhang, J. DNA binding, DNA cleavage and HSA interaction of several metal complexes containing N-(2-hydroxyethyl)-N′-benzoylthiourea and 1,10-phenanthroline ligands. J. Biol. Inorg. Chem. 2016, 21, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Joyner, J.C.; Reichfield, J.; Cowan, J.A. Factors Influencing the DNA Nuclease Activity of Iron, Cobalt, Nickel, and Copper Chelates. J. Am. Chem. Soc. 2011, 133, 15613–15626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.-D.; Huang, F.-P.; Chen, G.-J.; Gao, C.-Y.; Tian, J.-L.; Gu, W.; Liu, X.; Yan, S.-P. Four new copper(II) complexes with 1,3-tpbd ligand: Synthesis, crystal structures, magnetism, oxidative and hydrolytic cleavage of pBR322 DNA. J. Inorg. Biochem. 2010, 104, 431–441. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Liu, S.-D.; Deng, S.-Y.; Ji, L.-N.; Mao, Z.W. Cleavage of double-strand DNA by linear and triangular trinuclear copper complexes. J. Inorg. Biochem. 2006, 100, 1586–1593. [Google Scholar] [CrossRef]
- An, Y.; Tong, M.L.; Ji, L.N.; Mao, Z.W. Double-strand DNA cleavage by copper complexes of 2,2′-dipyridyl with electropositive pendants. Dalton Trans. 2006, 17, 2066–2071. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- Brabec, V.; Hrabina, O.; Kasparkova, J. Cytotoxic platinum coordination compounds. DNA binding agents. Coord. Chem. Rev. 2017, 351, 2–31. [Google Scholar] [CrossRef]
- Štarha, P.; Vancǒ, J.; Trávníček, Z. Platinum iodido complexes: A comprehensive overview of anticancer activity and mechanisms of action. Coord. Chem. Rev. 2019, 380, 103–135. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Hernandez, C.; Rodriguez, A.; Souza, P. Novel bis(thiosemicarbazones) of the 3,5-diacetyl-1,2,4-triazol series and their platinum(ii) complexes: Chemistry, antiproliferative activity and preliminary nephrotoxicity studies. Dalton Trans. 2011, 40, 5738–5745. [Google Scholar] [CrossRef] [Green Version]
- Barve, A.; Kumbhar, A.; Bhat, M.; Joshi, B.; Butcher, R.; Sonawane, U.; Joshi, R. Mixed-Ligand Copper(II) Maltolate Complexes: Synthesis, Characterization, DNA Binding and Cleavage, and Cytotoxicity. Inorg. Chem. 2009, 48, 9120–9132. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Ducharme, G.T.; Fischer, R.C.; Mautner, F.A.; Vančo, J.; Herchel, R.; Dvořák, Z.; Trávníček, Z. Copper(II) complexes based on tripodal pyrazolyl amines: Synthesis, structure, magnetic properties and anticancer activity. J. Inorg. Biochem. 2018, 180, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Herchel, R.; Dvořák, Z.; Trávníček, Z.; Mikuriya, M.; Louka, F.R.; Mautner, F.A.; Massoud, S.S. Cobalt(II) and copper(II) covalently and non-covalently dichlorido-bridged complexes of an unsymmetrical tripodal pyrazolyl-pyridyl amine ligand: Structures, magnetism and cytotoxicity. Inorg. Chim. Acta 2016, 451, 102–110. [Google Scholar] [CrossRef]
- Lu, J.; Sun, Q.; Li, J.-L.; Jiang, L.; Gu, W.; Liu, X.; Tian, J.-L.; Yan, S.-P. Two water-soluble copper(II) complexes: Synthesis, characterization, DNA cleavage, protein binding activities and in vitro anticancer activity studies. J. Inorg. Biochem. 2014, 137, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bavisotto, C.C.; Nikolic, D.; Gammaza, A.M.; Barone, R.; Cascio, F.L.; Mocciaro, E.; Zummo, G.; de Macairo, E.C.; Macario, A.J.; Cappelo, F.; et al. The dissociation of the Hsp60/pro-Caspase-3 complex by bis(pyridyl)oxadiazole copper complex (CubipyOXA) leads to cell death in NCI-H292 cancer cells. J. Inorg. Biochem. 2017, 170, 8–16. [Google Scholar] [CrossRef] [Green Version]
- de Medeiros, W.M.T.Q.; de Medeiros, M.J.C.; Carvalho, E.M.; de Lima, J.A.; da Oliveira, S.V.; de Pontes, B.A.C.F.; da Silva, F.O.N.; Ellena, J.A.; de Rocha, O.H.A.; de Sousa, E.H.S.; et al. A vanillin-based copper(II) metal complex with a DNA-mediated apoptotic activity. RSC Adv. 2018, 8, 16873–16886. [Google Scholar] [CrossRef] [Green Version]
- Erxleben, A. Interactions of copper complexes with nucleic acids. Chem. Rev. 2018, 360, 92–121. [Google Scholar] [CrossRef]
- Montagner, D.; Fresch, B.; Browne, K.; Gandian, V.; Erxleben, A. A Cu(II) complex targeting the translocator protein: In vitro and in vivo antitumor potential and mechanistic insights. Chem. Commun. 2017, 53, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Deka, B.; Sarkar, T.; Banerjee, S.; Kumar, A.; Mukherjee, S.; Deka, S.; Sailkia, K.K.; Hussain, A. Novel mitochondria targeted copper(II) complexes of ferrocenyl terpyridine and anticancer active 8-hydroxyquinolines showing remarkable cytotoxicity, DNA and protein binding affinity. Dalton Trans. 2017, 46, 396–409. [Google Scholar] [CrossRef]
- Dam, J.; Ismail, Z.; Kurebwa, T.; Ganger, N.; Harmse, L.; Marques, H.M.; Lemmerer, A.; Bode, M.L.; de Koning, C.B. Synthesis of copper and zinc 2-(pyridin-2-yl)imidazo[1,2-a]pyridine complexes and their potential anticancer activity. Eur. J. Med. Chem. 2017, 126, 353–368. [Google Scholar] [CrossRef]
- Kathiresan, S.; Mugesh, S.; Annaraj, J.; Murugan, M. Mixed-ligand copper(II) Schiff base complexes: The vital role of co-ligands in DNA/protein interactions and cytotoxicity. New J. Chem. 2017, 41, 1267–1283. [Google Scholar] [CrossRef]
- Jopp, M.; Becker, J.; Becker, S.; Miska, A.; Gandin, V.; Marzano, C.; Schindler, S. Anticancer activity of a series of copper(II) complexes with tripodal ligands. Eur. J. Med. Chem. 2017, 132, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, V.; Karthik, R.; Palaniandavar, M.; Periasamy, V.S.; Akbarsha, M.A.; Srinag, B.S.; Krishnamurthy, H. Mixed-Ligand Copper(II)-phenolate Complexes: Effect of Coligand on Enhanced DNA and Protein Binding, DNA Cleavage, and Anticancer Activity. Inorg. Chem. 2007, 46, 8208–8221. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Rijin, J.V.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Halfen, J.A.; Uhan, J.M.; Fox, D.C.; Mehn, M.P.; Que, L., Jr. Copper(II) Complexes of Pyridyl-Appended Diazacycloalkanes: Synthesis, Characterization, and Application to Catalytic Olefin Aziridination. Inorg. Chem. 2000, 39, 4913–4920. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Wilkinson, G.; Gillard, R.D.; McCleverty, J.A. (Eds.) Comprehensive Coordiqnation Chemistry; Pergamon Press: Oxford, UK, 1987; Volume 5, p. 533. [Google Scholar]
- Mautner, F.A.; Louka, F.R.; LeGuet, T.; Massoud, S.S. Pseudohalide copper(II) complexes derived from polypyridyl ligands: Synthesis and characterization. J. Mol. Struct. 2009, 919, 196–203. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Torvisco, A.; Henary, M.M.; Milner, A.; DeVillier, H.; Karsili, T.N.V.; Louka, F.R.; Massoud, S.S. Steric Effects of Alkyl Substituents at N-Donor Bidentate Amines Direct the Nuclearity, Bonding and Bridging Modes in Isothiocyanato-Copper(II) Coordination Compounds. Crystals 2019, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Mautner, F.A.; Albering, J.H.; Vicente, R.; Louka, F.R.; Gallo, A.A.; Massoud, S.S. Copper(II) complexes derived from tripodal tris[(2-ethyl-(1-pyrazolyl)]amine. Inorg. Chim. Acta 2011, 365, 290–296. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Pérez, V.; Monsalvo, I.; Demare, P.; Gómez-Vidales, V.; Regla, I.; Castillo, I. Dicopper(II) complexes of chiral C2-symmetric diamino-bis(2-methylpyridyl) and diamino-bis(2-methylbenzimidazolyl) ligands. Inorg. Chem. Comm. 2011, 14, 389–391. [Google Scholar] [CrossRef]
- Fry, F.H.; Fischmann, A.J.; Belousoff, M.J.; Spiccia, L.; Brügger, J. Kinetics and Mechanism of Hydrolysis of a Model Phosphate Diester by [Cu(Me3tacn)(OH2)2]2+ (Me3tacn = 1,4,7-Trimethyl-1,4,7-triazacyclononane). Inorg. Chem. 2005, 44, 941–950. [Google Scholar] [CrossRef]
- Li, F.-Z.; Feng, F.; Yu, L.; Xie, J.-q. The Metallomicelle of Lanthanide Metal (Ce, La) Aza-Macrocyclic Complexes with a Carboxyl Branch: The Catalytic Activity and Mechanism in the Hydrolysis of a Phosphate Diester. Solut. Chem. 2014, 43, 1331–1343. [Google Scholar] [CrossRef]
- Leichnitz, S.; Heinrich, J.; Kulak, N. A fluorescence assay for the detection of hydrogen peroxide and hydroxyl radicals generated by metallonucleases. Chem. Commun. 2018, 54, 13411–13414. [Google Scholar] [CrossRef]
- Zhao, G.; Chasteen, N.D. Oxidation of Good’s buffers by hydrogen peroxide. Anal. Biochem. 2006, 349, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.; Stubbe, J.; Kulak, N. Cu(II) complexes with hydrazone-functionalized phenanthrolines as self-activating metallonucleases. Inorg. Chim. Acta 2018, 481, 79–86. [Google Scholar] [CrossRef]
- Puckett, C.A.; Ernsta, R.J.; Barton, J.K. Exploring the cellular accumulation of metal complexes. Dalton Trans. 2010, 39, 1159–1170. [Google Scholar] [CrossRef] [Green Version]
- Serda, M.; Kalinowski, D.S.; Mrozek-Wilczkiewicz, A.; Musiol, R.; Szurko, A.; Ratuszn, A.; Pantarat, N.; Kovacevic, Z.; Merlot, A.M.; Richardson, D.R.; et al. Synthesis and characterization of quinoline-based thiosemicarbazones and correlation of cellular iron-binding efficacy to anti-tumor efficacy. Bioorg. Med. Chem. Lett. 2012, 22, 5527–5531. [Google Scholar] [CrossRef]
- Hertzberg, R.P.; Dervan, P.B. Cleavage of DNA with methidiumpropyl-EDTA-iron(II): Reaction conditions and product analyses. Biochemistry 1984, 23, 3934–3945. [Google Scholar] [CrossRef]
- Stöbener, D.D.; Uckert, M.; Cuellar-Camacho, J.L.; Hoppensack, A.; Weinhart, M. Ultrathin Poly(glycidyl ether) Coatings on Polystyrene for Temperature-Triggered Human Dermal Fibroblast Sheet Fabrication. ACS Biomater. Sci. Eng. 2017, 3, 2155–2165. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Tusa, A.F.; Bordelon, N.E.; Fischer, R.C.; Mautner, F.A.; Vančo, J.; Hošek, J.; Dvořákd, Z.; Trávníček, Z. Copper(II) complexes based on tripodal pyridyl amine derivatives as efficient anticancer agents. New J. Chem. 2019, 43, 6186–6196. [Google Scholar] [CrossRef]
- He, J.; Sun, J.; Mao, Z.W.; Ji, L.N.; Sun, H.Z. Phosphodiester hydrolysis and specific DNA binding and cleavage promoted by guanidinium-functionalized zinc complexes. J. Inorg. Biochem. 2009, 103, 851–858. [Google Scholar] [CrossRef]
- Dhar, S.; Reddy, P.A.N.; Chakravarty, A.R. Intramolecular nucleophilic activation promoting efficient hydrolytic cleavage of DNA by (aqua)bis(dipyridoquinoxaline)copper(II) complex. Dalton Trans. 2004, 5, 697–698. [Google Scholar] [CrossRef]
- Gupta, T.; Dhar, S.; Nethaji, M.; Chakravarty, A.R. Bis(dipyridophenazine)copper(II) complex as major groove directing synthetic hydrolase. Dalton Trans. 2004, 1896–1900. [Google Scholar] [CrossRef] [PubMed]










| [Cu(L)X]ClO4/PF6 | λmax (εmax, M−1cm−1) | ΛM (Ω−1·cm2·mol−1) | ΛM (Ω−1·cm2·mol−1) | |
|---|---|---|---|---|
| CH3CN | CH3CN | (CH3CN/H2O 1:1 v/v) | ||
| [Cu(L1)ClO4]ClO4 | (1-ClO4) | 645 (310) | 300 | 213 |
| [Cu(L2)ClO4]ClO4 | (2-ClO4) | ~752 (299) | 297 | 189 |
| [Cu(L3)ClO4]ClO4 | (3-ClO4) | ~565 (sh), ~618 (sh), 745 (226) | 301 | 188 |
| [Cu(L6)ClO4]ClO4 | (6-ClO4) | 583 (237) | 304 | 184 |
| [Cu(L1)Cl]PF6 | (1-Cl) | 770 (304) | 160 | 171 |
| [Cu(L4)Cl]ClO4·½H2O | (4-Cl) | 771 (324) | 145 | 168 |
| [Cu(L5)Cl]PF6 | (5-Cl) | 765 (352) | 162 | 177 |
| Complex | c (µM) | kobs (min−1) | kcat (h−1) | KM (M) | kcat/KM | Rate Enhancement (b) |
|---|---|---|---|---|---|---|
| 1-ClO4 | 60 | 2.65 × 10−5 | 7.79 × 10−1 | 5.03 × 10−4 | 5.58 × 106 (a) | 2.2 × 107 |
| 120 | 3.05 × 10−5 | |||||
| 180 | 3.91 × 10−5 | |||||
| 300 | 8.99 × 10−5 | |||||
| 420 | 2.02 × 10−4 | |||||
| 540 | 2.47 × 10−4 | |||||
| 1-Cl | 60 | 2.44 × 10−5 | 8.23 × 10−1 | 5.89 × 10−4 | 5.03 × 106 | 2.3 × 107 |
| 120 | 2.62 × 10−5 | |||||
| 180 | 4.11 × 10−5 | |||||
| 300 | 9.36 × 10−5 | |||||
| 420 | 1.63 × 10−4 | |||||
| 540 | 2.34 × 10−4 | |||||
| 2-ClO4 | 60 | 1.62 × 10−5 | 9.75 × 10−2 | 4.41 × 10−5 | 7.95 × 106 | 2.7 × 106 |
| 120 | 1.86 × 10−5 | |||||
| 180 | 2.02 × 10−5 | |||||
| 300 | 2.25 × 10−5 | |||||
| 420 | 2.58 × 10−5 | |||||
| 540 | 2.81 × 10−5 | |||||
| 3-ClO4 | 60 | 1.84 × 10−5 | 1.55 × 10−1 | 9.72 × 10−5 | 5.72 × 106 | 4.3 × 106 |
| 120 | 1.96 × 10−5 | |||||
| 180 | 2.05 × 10−5 | |||||
| 300 | 3.44 × 10−5 | |||||
| 420 | 4.57 × 10−5 | |||||
| 540 | 4.77 × 10−5 |
| Complex | IC50 Value (µM) in A2780 Cells | IC50 Value (µM) in Fibroblasts |
|---|---|---|
| 1-ClO4 | 38.7 (27.7–53.9) | 66.4 (44.1–99.1) |
| 1-Cl | >100 | >100 |
| 2-ClO4 | >100 | >100 |
| 3-ClO4 | 33.6 (18.1–62.6) | >100 |
| 4-Cl | >100 | >100 |
| 5-Cl | 31.2 (18.1–53.9) | 90.7 (51.0–161.5) |
| 6-ClO4 | >100 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doniz Kettenmann, S.; Nossol, Y.; Louka, F.R.; Legrande, J.R.; Marine, E.; Fischer, R.C.; Mautner, F.A.; Hergl, V.; Kulak, N.; Massoud, S.S. Copper(II) Complexes with Tetradentate Piperazine-Based Ligands: DNA Cleavage and Cytotoxicity. Inorganics 2021, 9, 12. https://doi.org/10.3390/inorganics9020012
Doniz Kettenmann S, Nossol Y, Louka FR, Legrande JR, Marine E, Fischer RC, Mautner FA, Hergl V, Kulak N, Massoud SS. Copper(II) Complexes with Tetradentate Piperazine-Based Ligands: DNA Cleavage and Cytotoxicity. Inorganics. 2021; 9(2):12. https://doi.org/10.3390/inorganics9020012
Chicago/Turabian StyleDoniz Kettenmann, Sebastian, Yvonne Nossol, Febee R. Louka, Julia R. Legrande, Elise Marine, Roland C. Fischer, Franz A. Mautner, Vinja Hergl, Nora Kulak, and Salah S. Massoud. 2021. "Copper(II) Complexes with Tetradentate Piperazine-Based Ligands: DNA Cleavage and Cytotoxicity" Inorganics 9, no. 2: 12. https://doi.org/10.3390/inorganics9020012
APA StyleDoniz Kettenmann, S., Nossol, Y., Louka, F. R., Legrande, J. R., Marine, E., Fischer, R. C., Mautner, F. A., Hergl, V., Kulak, N., & Massoud, S. S. (2021). Copper(II) Complexes with Tetradentate Piperazine-Based Ligands: DNA Cleavage and Cytotoxicity. Inorganics, 9(2), 12. https://doi.org/10.3390/inorganics9020012