Antigingivitis, Desensitizing, and Antiplaque Effects of Alkaline Toothpastes: A Randomized Clinical Trial
Abstract
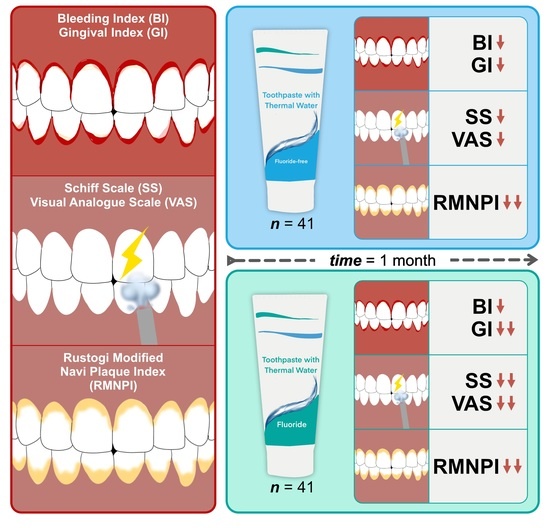
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Sampling Criteria
2.3.1. Inclusion Criteria
- Age 20–25 years;
- Signed an approved Informed Consent Form, authorizing the participation in the trial and use of the results of the trial for education purposes and publication;
- Clinically diagnosed gingivitis;
- At least one tooth with clinically diagnosed DH.
2.3.2. Exclusion Criteria
- Medical and pharmacotherapeutic histories that may compromise the protocol (pregnancy or breastfeeding, psychiatric disorders, allergies to toothpaste ingredients, eating disorders, etc.);
- Systemic conditions that are etiologic to DH (e.g., chronic acid regurgitation);
- History of chemotherapy or radiotherapy;
- Antibiotic, anti-inflammatory, or anti-coagulant therapy 4 weeks prior to or after the baseline visit;
- An oral mucosa pathology;
- Periodontal surgery within the previous 3 months;
- Orthodontic treatment within the previous 3 months;
- Any other pathology or teeth defects accompanied by pain;
- Use of any other agents for DH management 4 weeks prior to or after the baseline visit;
- Teeth with large restorations and/or teeth with restorations in the cervical area;
- Dental bleaching within the previous 3 months;
- Withdrawal of consent;
- Noncompliance with the study procedures;
- An adverse event that required treatment discontinuation.
2.4. Randomization
2.5. Interventions
2.6. Outcomes
2.6.1. MGI
- 0—Absence of inflammation;
- 1—Mild inflammation; slight change in color; little change in texture of any portion of the marginal or papillary gingival unit;
- 2—Mild inflammation; criteria as above plus the entire marginal or papillar gingival unit;
- 3—Moderate inflammation; glazing, redness, edema, and/or hypertrophy of the marginal or papillary gingival unit;
- 4—Severe inflammation; marked redness, edema and/or hypertrophy of the marginal or papillary gingival unit, spontaneous bleeding, congestion, or ulceration.
2.6.2. BI
- 0—No bleeding after 30 s;
- 1—Bleeding upon probing after 30 s;
- 2—Immediate bleeding.
2.6.3. Sensitivity Testing
- 0—No reaction;
- 1—Discomfort, but the patient does not insist on stopping the test;
- 2—Discomfort accompanied by a request to discontinue the test;
- 3—Severe pain and pronounced motor response, which meant that the test was immediately discontinued.
2.6.4. Salivary pH
2.6.5. RMNPI
2.6.6. Toothpaste pH
2.7. Statistical Analysis
2.8. Data Management
3. Results
3.1. Patient Flow and Demographics
3.2. Gingival Condition Assessment
3.3. Dentin Hypersensitivity Assessment
3.4. Oral Hygiene Assessment
3.5. Salivary pH Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Sotozono, M.; Ohkura, N.; Noiri, Y. Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects. Antibiotics 2022, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Contemporary perspective on plaque control. Br. Dent. J. 2012, 212, 601–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arweiler, N.B.; Netuschil, L. The oral microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef]
- Giuca, M.R.; Lardani, L.; Ligori, S.; Carli, E.; Giuca, G.; Miceli, M. Oral manifestations in paediatric patients with hepatobiliary diseases: A review. J. Biol. Regul. Homeost. Agents 2021, 35, 117–125. [Google Scholar] [CrossRef]
- Marsh, P.D.; Devine, D.A. How is the development of dental biofilms influenced by the host? J. Clin. Periodontol. 2011, 38, 28–35. [Google Scholar] [CrossRef]
- Kara, C.; Gökmenoglu, C.; Sahin, O.; Cinel, S.; Kara, N.B.; Sadik, E. A new management strategy for the treatment of streptococcal gingivitis: A pilot study. J. Pak. Med. Assoc. 2018, 68, 235–239. [Google Scholar]
- Koppolu, P.; Sirisha, S.; Penala, S.; Reddy, P.K.; Alotaibi, D.H.; Abusalim, G.S.; Lingam, A.S.; Mukhtar, A.H.; Barakat, A.; Almokhatieb, A.A. Correlation of Blood and Salivary pH Levels in Healthy, Gingivitis, and Periodontitis Patients before and after Non-Surgical Periodontal Therapy. Diagnostics 2022, 12, 97. [Google Scholar] [CrossRef]
- Kado, I.; Kunimatsu, R.; Yoshimi, Y.; Medina, C.C.; Yamada, S.; Tanimoto, K. Surveillance of salivary properties of pre-orthodontic patients in relation to age and sex. Sci. Rep. 2021, 11, 655. [Google Scholar] [CrossRef]
- Foglio-Bonda, P.L.; Migliario, M.; Rocchetti, V.; Pattarino, F.; Foglio-Bonda, A. Daily and annually variation of unstimulated whole saliva flow rate and pH and their relation with body profile in healthy young adults. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2538–2545. [Google Scholar]
- Paszyńska, E.; Słopień, A.; Slebioda, Z.S.; Dyszkiewicz-Konwińska, M.; Weglarz, M.; Rajewski, A. Macroscopic evaluation of the oral mucosa and analysis of salivary pH in patients with anorexia nervosa. Psychiatr. Pol. 2014, 48, 453–464. [Google Scholar] [PubMed]
- Caruso, A.A.; Del Prete, S.; Ferrara, L.; Serra, R.; Telesca, D.A.; Ruggiero, S.; Russo, T.; Sivero, L. Relationship between gastroesophageal reflux disease and Ph nose and salivary: Proposal of a simple method outpatient in patients adults. Open Med. 2016, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, S.; Jalihal, U.; Devi, Y.; Rakesh, N.; Chauhan, P.; Sharma, S. Oral pH in gastroesophageal reflux disease. Indian J. Gastroenterol. 2016, 35, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Bechir, F.; Pacurar, M.; Tohati, A.; Bataga, S.M. Comparative Study of Salivary pH, Buffer Capacity, and Flow in Patients with and without Gastroesophageal Reflux Disease. Int. J. Environ. Res. Public Health 2021, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Seethalakshmi, C.; Jagat Reddy, R.C.; Asifa, N.; Prabhu, S. Correlation of Salivary pH, Incidence of Dental Caries and Periodontal Status in Diabetes Mellitus Patients: A Cross-sectional Study. J. Clin. Diagn. Res. 2016, 10, ZC12–ZC14. [Google Scholar] [CrossRef]
- Marques, R.C.R.; da Silva, J.R.; Vieira Lima, C.P.; Stefani, C.M.; Damé-Teixeira, N. Salivary parameters of adults with diabetes mellitus: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 176–189. [Google Scholar] [CrossRef]
- Saluja, P. Comparative Evaluation of the Effect of Menstruation, Pregnancy and Menopause on Salivary Flow Rate, pH and Gustatory Function. J. Clin. Diagn. Res. 2014, 8, ZC81–ZC85. [Google Scholar] [CrossRef]
- Jain, K.; Kaur, H. Prevalence of oral lesions and measurement of salivary pH in the different trimesters of pregnancy. Singap. Med. J. 2015, 56, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Foglio-Bonda, P.L.; Rocchetti, V.; Nardella, A.; Fantinato, M.; Sandhu, K.; Foglio-Bonda, A. Salivary pH and flow rate in menopausal women. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 918–922. [Google Scholar] [CrossRef]
- Migliario, M.; Bindi, M.; Surico, D.; De Pedrini, A.; Minsenti, S.; Pezzotti, F.; Mele, B.; Foglio Bonda, P.L. Changes in salivary flow rate and pH in pregnancy. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1804–1810. [Google Scholar] [CrossRef]
- Mishra, R.; Haider, K.; Rizwan, R.; Monga, S.; Pritam, A.; Singh, P. Assessment of Effect of Menopause on Saliva and Oral Health Status. J. Pharm. Bioallied Sci. 2021, 13, S1535–S1537. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Schachtele, C.F. Effect of pH on the Growth and Proteolytic Activity of Porphyromonas gingivalis and Bacteroides intermedius. J. Dent. Res. 1990, 69, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Saito, K.; Schachtele, C.F.; Yamada, T. Acid tolerance and acid-neutralizing activity of Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum. Oral Microbiol. Immunol. 1997, 12, 323–328. [Google Scholar] [CrossRef]
- Shellis, R.P.; Addy, M. The interactions between attrition, abrasion and erosion in tooth wear. Monogr. Oral Sci. 2014, 25, 32–45. [Google Scholar] [CrossRef]
- Martins, V.; Da Costa Ramos, R.; Pimenta Lima, M.; Correia De Ara, R.; Cavalcanti, A. Effect of surface protection on the permeability of eroded dentin. J. Conserv. Dent. 2018, 21, 16–20. [Google Scholar] [CrossRef]
- Yoshizaki, K.T.; Francisconi-dos-Rios, L.F.; Sobral, M.A.P.; Aranha, A.C.C.; Mendes, F.M.; Scaramucci, T. Clinical features and factors associated with non-carious cervical lesions and dentin hypersensitivity. J. Oral Rehabil. 2017, 44, 112–118. [Google Scholar] [CrossRef] [PubMed]
- West, N.X.; He, T.; Zou, Y.; DiGennaro, J.; Biesbrock, A.; Davies, M. Bioavailable gluconate chelated stannous fluoride toothpaste meta-analyses: Effects on dentine hypersensitivity and enamel erosion. J. Dent. 2021, 105, 103566. [Google Scholar] [CrossRef]
- Pretha, M.; Setty, S.; Ravindra, S. Dentinal hypersensitivity?—Can this agent be the solution? Indian J. Dent. Res. 2006, 17, 178–184. [Google Scholar] [CrossRef]
- Taani, D.Q.; Awartani, F. Prevalence and distribution of dentin hypersensitivity and plaque in a dental hospital population. Quintessence Int. 2001, 32, 372–376. [Google Scholar]
- Expert Committee of Dentin Hypersensitivity, Society of Preventive Dentistry, Chinese Stomatological Association. Guideline for diagnosis, prevention and treatment of dentin hypersensitivity. Zhonghua Kou Qiang Yi Xue Za Zhi 2019, 54, 223–227. [Google Scholar] [CrossRef]
- Chen, L.; Al-Bayatee, S.; Khurshid, Z.; Shavandi, A.; Brunton, P.; Ratnayake, J. Hydroxyapatite in Oral Care Products—A Review. Materials 2021, 14, 4865. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, M.; Sokhova, I.; Doroshina, V.; Arakelyan, M.; Novozhilova, N.; Babina, K. The Effect of Toothpastes Containing Hydroxyapatite, Fluoroapatite, and Zn-Mg-hydroxyapatite Nanocrystals on Dentin Hypersensitivity: A Randomized Clinical Trial. J. Int. Soc. Prev. Community Dent. 2022, 12, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Valenti, C.; Pagano, S.; Bozza, S.; Ciurnella, E.; Lomurno, G.; Capobianco, B.; Coniglio, M.; Cianetti, S.; Marinucci, L. Use of the Er:YAG laser in conservative dentistry: Evaluation of the microbial population in carious lesions. Materials 2021, 14, 2387. [Google Scholar] [CrossRef]
- Polyakova, M.; Arakelyan, M.; Babina, K.; Margaryan, E.; Sokhova, I.; Doroshina, V.; Novozhilova, N. Qualitative and Quantitative Assessment of Remineralizing Effect of Prophylactic Toothpaste Promoting Brushite Formation: A Randomized Clinical Trial. J. Int. Soc. Prev. Community Dent. 2020, 10, 359–367. [Google Scholar] [CrossRef]
- Martins, C.C.; Firmino, R.T.; Riva, J.J.; Ge, L.; Carrasco-Labra, A.; Brignardello-Petersen, R.; Colunga-Lozano, L.E.; Granville-Garcia, A.F.; Costa, F.O.; Yepes-Nuñez, J.J.; et al. Desensitizing Toothpastes for Dentin Hypersensitivity: A Network Meta-analysis. J. Dent. Res. 2020, 99, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Fambrini, E.; Miceli, M.; Pasini, M.; Giuca, M.R. Clinical Evaluation of the Use of Desensitizing Agents in the Management of Dentinal Hypersensitivity. Appl. Sci. 2022, 12, 11238. [Google Scholar] [CrossRef]
- Orchardson, R.; Gillam, D.G. Managing dentin hypersensitivity. J. Am. Dent. Assoc. 2006, 137, 990–998. [Google Scholar] [CrossRef]
- Viana, Í.E.L.; Lopes, R.M.; Silva, F.R.O.; Lima, N.B.; Aranha, A.C.C.; Feitosa, S.; Scaramucci, T. Novel fluoride and stannous—Functionalized β-tricalcium phosphate nanoparticles for the management of dental erosion. J. Dent. 2020, 92, 103263. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Gargani, A.; Parcianello, R.G.; Pezzato, L.; Bertolini, R.; Zuccon, A.; Stellini, E.; Ludovichetti, F.S. Protection against Dental Erosion and the Remineralization Capacity of Non-Fluoride Toothpaste, Fluoride Toothpaste and Fluoride Varnish. Appl. Sci. 2023, 13, 1849. [Google Scholar] [CrossRef]
- Lammers, P.C.; Borggreven, J.M.P.M.; Driessens, F.C.M. Influence of fluoride and pH on in vitro remineralization of bovine enamel. Caries Res. 1992, 26, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Margolis, H.C. Enhanced enamel remineralization under acidic conditions in vitro. J. Dent. Res. 2008, 87, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Sewón, L.; Söderling, E.; Karjalainen, S. Comparative study on mineralization-related intraoral parameters in periodontitis-affected and periodontitis-free adults. Scand. J. Dent. Res. 1990, 98, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, K.; Zareena; Hegde, S.; Arun Kumar, M.S. Assessment of salivary calcium, phosphate, magnesium, pH, and flow rate in healthy subjects, periodontitis, and dental caries. Contemp. Clin. Dent. 2015, 6, 461. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Varma, S.; Suragimath, G.; Zope, S. Estimation and Comparison of Salivary Calcium, Phosphorous, Alkaline Phosphatase and pH Levels in Periodontal Health and Disease: A Cross-sectional Biochemical Study. J. Clin. Diagn. Res. 2016, 10, ZC58–ZC61. [Google Scholar] [CrossRef] [PubMed]
- Al Habashneh, R.; Farasin, R.; Khader, Y. The effect of a triclosan/copolymer/fluoride toothpaste on plaque formation, gingivitis, and dentin hypersensitivity: A single-blinded randomized clinical study. Quintessence Int. (Berl.) 2017, 48, 123–130. [Google Scholar] [CrossRef]
- Monterubbianesi, R.; Sparabombe, S.; Tosco, V.; Profili, F.; Mascitti, M.; Hosein, A.; Putignano, A.; Orsini, G. Can Desensitizing Toothpastes Also Have an Effect on Gingival Inflammation? A Double-Blind, Three-Treatment Crossover Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 8927. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef]
- Rustogi, K.; Curtis, J.; Volpe, A.; Kemp, J.; McCool, J.; Korn, L. Refinement of the Modified Navy Plaque Index to increase plaque scoring efficiency in gumline and interproximal tooth areas. J. Clin. Dent. 1992, 3 (Suppl. C), C9–C12. [Google Scholar]
- Gavic, L.; Gorseta, K.; Borzabadi-Farahani, A.; Tadin, A.; Glavina, D. Influence of Toothpaste pH on Its Capacity to Prevent Enamel Demineralization. Contemp. Clin. Dent. 2017, 9, 554–559. [Google Scholar] [CrossRef]
- Schemehorn, B.R.; Moore, M.H.; Putt, M.S. Abrasion, polishing, and stain removal characteristics of various commercial dentifrices in vitro. J. Clin. Dent. 2011, 22, 11–18. [Google Scholar]
- Arnold, W.H.; Gröger, C.; Bizhang, M.; Naumova, E.A. Dentin abrasivity of various desensitizing toothpastes. Head Face Med. 2016, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myneni, S.R. Effect of baking soda in dentifrices on plaque removal. J. Am. Dent. Assoc. 2017, 148, S4–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newbrun, E. Cariology; Quentessence Publishing, Co.: Chicago, IL, USA, 1989; 389p. [Google Scholar]
- van Loveren, C. Exposed cervical dentin and dentin hypersensitivity summary of the discussion and recommendations. Clin. Oral Investig. 2013, 17 (Suppl. 1), 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Cabezas, C.; Hara, A.T.; Hefferren, J.; Lippert, F. Abrasivity testing of dentifrices—Challenges and current state of the art. Monogr. Oral Sci. 2013, 23, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hamza, B.; Attin, T.; Cucuzza, C.; Gubler, A.; Wegehaupt, F.J. RDA and REA Values of Commercially Available Toothpastes Utilising Diamond Powder and Traditional Abrasives. Oral Health Prev. Dent. 2020, 18, 807–814. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.; Truong, V.M.; Lee, J.W.; Park, Y.S. Is whitening toothpaste safe for dental health?: RDA-PE method. Dent. Mater. J. 2022, 41, 731–740. [Google Scholar] [CrossRef]
- Owens, J.; Addy, M.; Faulkner, J. An 18-week home-use study comparing the oral hygiene and gingival health benefits of triclosan and fluoride toothpastes. J. Clin. Periodontol. 1997, 24, 626–631. [Google Scholar] [CrossRef]
- Petersson, L.G. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin. Oral Investig. 2013, 17, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J. Can. Dent. Assoc. 2003, 69, 221–226. [Google Scholar]
- Lussi, A.; Megert, B.; Eggenberger, D.; Jaeggi, T. Impact of Different Toothpastes on the Prevention of Erosion. Caries Res. 2008, 42, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.; Gross, K.B.; Overman, P.R.; Anderson, D. Effectiveness of chlorhexidine and sodium fluoride in reducing dentin hypersensitivity. J. Dent. Hyg. JDH 1991, 65, 340–344. [Google Scholar] [PubMed]
- Lăzureanu, P.C.; Popescu, F.; Tudor, A.; Stef, L.; Negru, A.G.; Mihăilă, R. Saliva pH and Flow Rate in Patients with Periodontal Disease and Associated Cardiovascular Disease. Med. Sci. Monit. 2021, 27, e931362. [Google Scholar] [CrossRef] [PubMed]
- Galgut, P. The relevance of pH to gingivitis and periodontitis. J. Int. Acad. Periodontol. 2001, 3, 61–67. [Google Scholar] [PubMed]
- Orozco Páez, J.; Contreras de la Rosa, L.; López Banda, J. Salivary pH as an improvement parameter in patients with periodontitis: A pilot study. Cienc. Innovación Salud 2020, 277–285, e85. [Google Scholar] [CrossRef]
- García Linares, S.; Bravo Castañola, F.; Ayala Luis, J.; Bardales Cuzquén, G. pH en saliva total en pacientes con enfermedad periodontal del Servicio de Periodoncia de la Facultad de Odontología de la UNMSM. Odontol. Sanmarquina 2014, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Setiawan, S.; Haroen, E.R.; Hadidjah, D. The difference in saliva pH before and after brushing with fluoride containing toothpaste and without toothpaste. Padjadjaran J. Dent. 2008, 20, 139–142. [Google Scholar] [CrossRef] [Green Version]
- Fibryanto, E.; Widyastuti, W. Effect of brushing the teeth before and after meals on salivary pH: A quasi-experimental study. J. Int. Oral Health 2022, 14, 163. [Google Scholar] [CrossRef]

| Group | Toothpaste Composition | Active Ingredient Description/RDA/pH |
|---|---|---|
| TW | Aqua (Castéra-Verduzan Thermal Spring water), Glycerin, Hydrogenated Starch Hydrolysate, Aqua, Hydrated Silica, Decyl Glucoside, Cellulose Gum Aroma, Sodium Benzoate, Stevia Rebaudiana Extract, Sodium Hydroxide, Limonene. | Castéra-Verduzan Thermal Spring water RDA 7 pH = 8.8 ± 0.2 |
| TWF | Aqua (Castéra-Verduzan Thermal Spring water), Glycerin, Hydrogenated Starch Hydrolysate, Aqua, Hydrated Silica, Decyl Glucoside, Cellulose Gum Aroma, Sodium Benzoate, Sodium Fluoride, Stevia Rebaudiana Extract, Sodium Hydroxide, Limonene, Sodium Fluoride: 1450 ppm. | Castéra-Verduzan Thermal Spring water, Sodium Fluoride: 1450 ppm RDA 17 pH = 8.8 ± 0.1 |
| Tested Toothpaste | TW | TWF | p Value |
|---|---|---|---|
| Sex n (%) | |||
| Female | 30 (73.2) | 34 (82.9) | 0.4241 1 |
| Male | 11 (26.8) | 7 (17.07) | |
| Total | 41 (100) | 41 (100) | |
| Age | |||
| Mean (sd) | 21.39 (1.53) | 21.05 (1.34) | 0.2498 2 |
| Median (Q1, Q3) | 21 (20, 22) | 21 (20, 21) | |
| Min, Max | 20, 25 | 20, 25 |
| Group | Baseline | 4 Weeks | Effect Size (Cohen’s d) |
|---|---|---|---|
| TW (n = 41) | |||
| Mean (SD) | 0.053 (0.029) a | 0.030 (0.016) A | 0.99 |
| CI95% | 0.044–0.062 | 0.025–0.035 | 0.52–1.46 |
| Median (Q1, Q3) | 2.86 (1.93; 3.43) | 0.63 (0.43; 0.78) | |
| p values | p < 0.001 1 | ||
| TWF (n = 41) | |||
| Mean (SD) | 0.053 (0.024) a | 0.017 (0.013) B | 1.71 |
| CI95% | 0.043–0.058 | 0.013–0.021 | 1.18–2.24 |
| Median (Q1, Q3) | 2.46 (1.96; 3.21) | 0.54 (0.43; 0.86) | |
| p values | p < 0.001 1 | ||
| Group | Baseline | 4 Weeks | Effect Size (Cohen’s d) |
|---|---|---|---|
| TW (n = 41) | |||
| Mean (SD) | 0.21 (0.07) a | 0.05 (0.02) A | 3.17 |
| CI95% | 0.19–0.23 | 0.05–0.06 | 2.39–3.94 |
| Median (Q1, Q3) | 0.22 (0.17; 0.26) | 0.05 (0.04; 0.07) | |
| p values | p < 0.001 1 | ||
| TWF (n = 41) | |||
| Mean (SD) | 0.20 (0.07) a | 0.05 (0.02) A | 2.64 |
| CI95% | 0.18–0.22 | 0.05–0.06 | 1.96–3.32 |
| Median (Q1, Q3) | 0.20 (0.14; 0.25) | 0.05 (0.04; 0.07) | |
| p values | p < 0.001 1 | ||
| Group | Baseline | 4 Weeks | Effect Size (Cohen’s d) |
|---|---|---|---|
| TW (n = 41) | |||
| Mean (SD) | 6.8 (2.5) a | 6.2 (2.6) A | 0.23 |
| CI95% | 6.0–7.5 | 5.4–7.0 | −0.21–0.66 |
| Median (Q1, Q3) | 7 (5; 9) | 7 (3; 8) | |
| p values | p = 0.00714 1 | ||
| TWF (n = 41) | |||
| Mean (SD) | 6.3 (2.2) a | 0.8 (0.9) B | 3.28 |
| CI95% | 5.6–7.0 | 0.5–1.0 | 2.51–4.04 |
| Median (Q1, Q3) | 6 (5; 8) | 1 (0; 1) | |
| p values | p < 0.001 1 | ||
| Group | Baseline | 4 Weeks | Effect Size (Cohen’s d) |
|---|---|---|---|
| TW (n = 41) | |||
| Mean (SD) | 2.2 (0.8) a | 1.2 (0.7) A | 1.30 |
| CI95% | 2.0–2.5 | 1.0–1.5 | 0.82–1.78 |
| Median (Q1, Q3) | 2 (2; 3) | 1 (1; 2) | |
| p values | p < 0.001 1 | ||
| TWF (n = 41) | |||
| Mean (SD) | 2.1 (0.8) a | 0.2 (0.4) B | 3.10 |
| CI95% | 1.9–2.3 | 0.1–0.3 | 2.40–3.80 |
| Median (Q1, Q3) | 2 (2; 3) | 0 (0; 0) | |
| p values | p < 0.001 1 | ||
| Score | TW (0 w) 1 | TW (4 w) 2 | TWF (0 w) | TWF (4 w) |
|---|---|---|---|---|
| 0 | - | - | - | 33 (80) |
| 1 | 9 (22) | 7 (17) | 10 (24) | 8 (20) |
| 2 | 13 (32) | 17 (41.5) | 17 (41) | - |
| 3 | 19 (46) | 17 (41.5) | 14 (34) | - |
| p < 0.001 3 | p < 0.001 3 | |||
| Group | Baseline | 4 Weeks | Effect Size (Cohen’s d) |
|---|---|---|---|
| TW (n = 41) | |||
| Mean (SD) | 2.85 (1.04) a | 0.62 (0.25) A | 2.76 |
| CI95% | 2.53–3.17 | 0.55–0.70 | 2.03–3.48 |
| Median (Q1, Q3) | 2.86 (1.93; 3.43) | 0.63 (0.43; 0.78) | |
| p values | p < 0.001 1 | ||
| TWF (n = 41) | |||
| Mean (SD) | 2.73 (1.07) a | 0.61 (0.21) A | 2.96 |
| CI95% | 2.41–3.06 | 0.55–0.68 | 2.20–3.70 |
| Median (Q1, Q3) | 2.46 (1.96; 3.21) | 0.54 (0.43; 0.86) | |
| p values | p < 0.001 1 | ||
| Group | Before | After | Effect Size (Cohen’s d) |
|---|---|---|---|
| TW (n = 41) | |||
| Mean (SD) | 7.07 (0.32) a | 7.36 (0.33) A | 0.89 |
| CI95% | 6.88–7.27 | 7.16–7.55 | 0.13–1.64 |
| Median (Q1, Q3) | 7.10 (7.00; 7.20) | 7.40 (7.05; 7.55) | |
| p values | p = 0.02275 1 | ||
| TWF (n = 41) | |||
| Mean (SD) | 6.99 (0.39) a | 7.50 (0.23) A | 1.59 |
| CI95% | 6.87–7.11 | 7.43–7.57 | 0.58–2.57 |
| Median (Q1, Q3) | 7.00 (6.80; 7.25) | 7.60 (7.30; 7.65) | |
| p values | p = 0.006558 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novozhilova, N.; Andreeva, E.; Polyakova, M.; Makeeva, I.; Sokhova, I.; Doroshina, V.; Zaytsev, A.; Babina, K. Antigingivitis, Desensitizing, and Antiplaque Effects of Alkaline Toothpastes: A Randomized Clinical Trial. Dent. J. 2023, 11, 96. https://doi.org/10.3390/dj11040096
Novozhilova N, Andreeva E, Polyakova M, Makeeva I, Sokhova I, Doroshina V, Zaytsev A, Babina K. Antigingivitis, Desensitizing, and Antiplaque Effects of Alkaline Toothpastes: A Randomized Clinical Trial. Dentistry Journal. 2023; 11(4):96. https://doi.org/10.3390/dj11040096
Chicago/Turabian StyleNovozhilova, Nina, Elena Andreeva, Maria Polyakova, Irina Makeeva, Inna Sokhova, Vladlena Doroshina, Alexandr Zaytsev, and Ksenia Babina. 2023. "Antigingivitis, Desensitizing, and Antiplaque Effects of Alkaline Toothpastes: A Randomized Clinical Trial" Dentistry Journal 11, no. 4: 96. https://doi.org/10.3390/dj11040096
APA StyleNovozhilova, N., Andreeva, E., Polyakova, M., Makeeva, I., Sokhova, I., Doroshina, V., Zaytsev, A., & Babina, K. (2023). Antigingivitis, Desensitizing, and Antiplaque Effects of Alkaline Toothpastes: A Randomized Clinical Trial. Dentistry Journal, 11(4), 96. https://doi.org/10.3390/dj11040096