Resistance of PETG Materials on Thermocycling and Brushing
Abstract
:1. Introduction
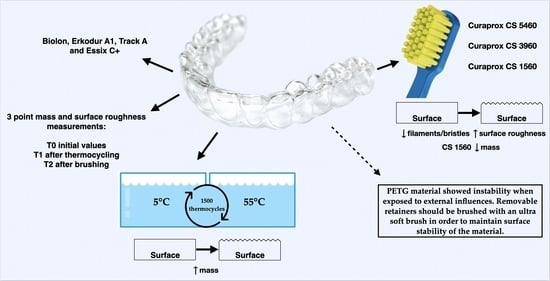
2. Materials and Methods
2.1. Specimen Preparation
2.2. Thermocycling
2.3. Brushing
2.4. Evaluation of Surface Roughness and Mass
2.5. Statistical Analysis
3. Results
3.1. Effect of Thermocycling on the Top Side
3.2. Effect of Brushing on the Top Side
3.3. Effect of Thermocycling on the Bottom Side
3.4. Effect of Brushing on the Bottom Side
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponitz, R.J. Invisible retainers. Am. J. Orthod. 1971, 59, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.J.; LeDoux, W.; McMinn, R. Essix retainers: Fabrication and supervision for permanent retention. J. Clin. Orthod. 1993, 27, 37–45. [Google Scholar] [PubMed]
- Gardner, G.D.; Dunn, W.J.; Taloumis, L. Wear comparison of thermoplastic materials used for orthodontic retainers. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.S.; Fang, D.Y.; Zhang, N.; Ding, X.J.; Zhang, K.Y.; Bai, Y.X. Mechanical Properties of Orthodontic Thermoplastics PETG/PC2858 after Blending. Chin. J. Dent. Res. 2016, 19, 43–48. [Google Scholar] [PubMed]
- Alexandropoulos, A.; Al Jabbari, Y.S.; Zinelis, S.; Eliades, T. Chemical and mechanical characteristics of contemporary thermoplastic orthodontic materials. Aust. Orthod. J. 2015, 31, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Zhang, N.; Chen, H.; Bai, Y. Dynamic stress relaxation of orthodontic thermoplastic materials in a simulated oral environment. Dent. Mater. J. 2013, 32, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Dupaix, R.B.; Boyce, M.C. Finite strain behavior of poly(ethylene terephthalate) (PET) and poly(ethylene terephthalate)-glycol (PETG). Polymers 2005, 46, 4827–4838. [Google Scholar] [CrossRef]
- Tsai, Y.; Fan, C.-H.; Hung, C.Y.; Tsai, F.J. Poly(ethylene Terephthalate) Copolymers That Contain 5-Tert-Butylisophthalic Acid and 1-3/1-4-Cyclohexanedimethanol: Synthesis, Characterization, and Properties. J. Appl. Polym. 2007, 104, 279–285. [Google Scholar] [CrossRef]
- Wang, K.; Shen, J.; Ma, Z.; Zhang, Y.; Xu, N.; Pang, S. Preparation and Properties of Poly(ethylene glycol-co-cyclohexane-1,4-dimethanol terephthalate)/Polyglycolic Acid (PETG/PGA) Blends. Polymers 2021, 13, 452. [Google Scholar] [CrossRef]
- Liu, F.; Wang, W.; Mirihanage, W.; Hinduja, S.; Bartolo, P.J. A plasma-assisted bioextrusion system for tissue engineering. CIRP Ann. 2018, 67, 229–232. [Google Scholar] [CrossRef]
- Kováčová, M.; Kozakovičová, J.; Procházka, M.; Janigová, I.; Vysopal, M.; Černičková, I.; Krajčovič, J.; Špitalský, Z. Novel Hybrid PETG Composites for 3D Printing. Appl. Sci. 2020, 10, 3062. [Google Scholar] [CrossRef]
- Li, Y.; Ma, T.; Yang, S.T.; Kniss, D.A. Thermal compression and characterization of three-dimensional nonwoven PET matrices as tissue engineering scaffolds. Biomaterials 2001, 22, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Omar, A.M.; Daskalakis, E.; Hou, Y.; Huang, B.; Strashnov, I.; Grieve, B.D.; Bártolo, P. The Potential of Polyethylene Terephthalate Glycol as Biomaterial for Bone Tissue Engineering. Polymers 2020, 12, 3045. [Google Scholar] [CrossRef] [PubMed]
- Latko, P.; Dydek, K.; Boczkowska, A. Thermal, Rheological and Mechanical Properties of PETG/RPETG Blends. J. Polym. Environ. 2019, 27, 2600–2606. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Irska, I.; Pawlikowska, D.; Piesowicz, E. Synthesis and Characterization of New Reactive Polymer Blends Based on Post-Consumer Glycol-Modified poly(ethylene Terephthalate) Foils and poly(tetramethylene Oxide). Polimery 2018, 63, 45–48. [Google Scholar] [CrossRef]
- Ruyi, W.; Zhihe, Z.; Yu, L. Current situation and prospect for orthodontic thermoplastic materials. West China J. Stomatol. 2018, 36, 87–91. [Google Scholar]
- Wang, F. A new thermoplastic retainer. J. Clin. Orthod. 1997, 31, 754–757. [Google Scholar]
- Ashari, A.; Nik Mustapha, N.M.; Yuen, J.J.X.; Saw, Z.K.; Lau, M.N.; Xian, L.; Syed Mohamed, A.M.F.; Megat Abdul Wahab, R.; Yeoh, C.K.; Deva Tata, M.; et al. A two-year comparative assessment of retention of arch width increases between modified vacuum-formed and Hawley retainers: A multi-center randomized clinical trial. Prog. Orthod. 2022, 23, 40. [Google Scholar] [CrossRef]
- Lyros, I.; Tsolakis, I.A.; Maroulakos, M.P.; Fora, E.; Lykogeorgos, T.; Dalampira, M.; Tsolakis, A.I. Orthodontic Retainers—A Critical Review. Children 2023, 10, 230. [Google Scholar] [CrossRef]
- Ahn, H.W.; Ha, H.R.; Lim, H.N.; Choi, S. Effects of aging procedures on the molecular, biochemical, morphological, and mechanical properties of vacuum-formed retainers. J. Mech. Behav. Biomed. Mater. 2015, 51, 356–366. [Google Scholar] [CrossRef]
- Jin, C.; Bennani, F.; Gray, A.; Farella, M.; Mei, L. Survival Analysis of Orthodontic Retainers. Eur. J. Orthod. 2018, 40, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.; Thanigaiyarasu, G.; Vani, K. Study on the Relationship between Surface Roughness of AA6061 Alloy End Milling and Image Texture Features of Milled Surface. Procedia Eng. 2014, 97, 150–157. [Google Scholar] [CrossRef]
- Porojan, L.; Vasiliu, R.D.; Porojan, S.D.; Bîrdeanu, M.I. Surface Quality Evaluation of Removable Thermoplastic Dental Appliances Related to Staining Beverages and Cleaning Agents. Polymers 2020, 12, 1736. [Google Scholar] [CrossRef] [PubMed]
- Adegbola, A.; Aghachi, I.E.A.; Sadiku-Agboola, O. SEM and AFM microscopical characterization of rPAN fibre and PET blends. Alex. Eng. J. 2018, 57, 475–481. [Google Scholar]
- Pascual, A.L.; Beeman, C.S. The Essential Work of Fracture of Thermoplastic Orthodontic Retainer Materials. Angle Orthod. 2010, 80, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Al Groosh, D.H.; Bozec, L.; Pratten, J.; Hunt, N.P. The influence of surface roughness and surface dynamics on the attachment of Methicillin-Resistant Staphylococcus aureus onto orthodontic retainer materials. Dent. Mater. J. 2015, 34, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.S.; Billington, R.W.; Pearson, G.J. The in Vivo Perception of Roughness of Restorations. Br. Dent. J. 2004, 196, 42–45. [Google Scholar] [CrossRef]
- Papadopoulou, A.K.; Cantele, A.; Polychronis, G.; Zinelis, S.; Eliades, T. Changes in Roughness and Mechanical Properties of Invisalign® Appliances after One- and Two-Weeks Use. Materials 2019, 12, 2406. [Google Scholar] [CrossRef]
- Akgün, F.A.; Şenışık, N.E.; Çetin, E.S. Evaluation of the Efficacy of Different Cleaning Methods for Orthodontic Thermoplastic Retainers in terms of Bacterial Colonization. Turk. J. Orthod. 2019, 32, 219–228. [Google Scholar] [CrossRef]
- Turkoz, C.; Canigur Bavbek, N.; Kale Varlik, S.; Akca, G. Influence of thermoplastic retainers on Streptococcus mutans and Lactobacillus adhesion. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 598–603. [Google Scholar] [CrossRef]
- Eichenauer, J.; Serbesis, C.; Ruf, S. Cleaning Removable Orthodontic Appliances—A Survey. J. Orofac. Orthop. 2011, 72, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Šimunović, L.; Blagec, T.; Meštrović, S. The Influence of Different Cleaning Protocols on the Surface Roughness of Orthodontic Retainers. Appl. Sci. 2023, 13, 1319. [Google Scholar] [CrossRef]
- Wible, E.; Agarwal, M.; Altun, S.; Ramir, T.; Viana, G.; Evans, C.; Lukic, H.; Megremis, S.; Atsawasuwan, P. Long-term effects of different cleaning methods on copolyester retainer properties. Angle Orthod. 2019, 89, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Wible, E.; Ramir, T.; Altun, S.; Viana, G.; Evans, C.; Lukic, H.; Megremis, S.; Atsawasuwan, P. Long-term effects of seven cleaning methods on light transmittance, surface roughness, and flexural modulus of polyurethane retainer material. Angle Orthod. 2018, 88, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, R.D.; Porojan, S.D.; Bîrdeanu, M.I.; Porojan, L. Effect of Thermocycling, Surface Treatments and Microstructure on the Optical Properties and Roughness of CAD-CAM and Heat-Pressed Glass Ceramics. Materials 2020, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, B.A.; Willmann, J.H.; Nimer, A.; Drescher, D. Effect of in Vitro Aging by Water Immersion and Thermocycling on the Mechanical Properties of PETG Aligner Material. J. Orofac. Orthop. 2019, 80, 292–303. [Google Scholar] [CrossRef]
- Gale, M.S.; Darvell, B.W. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- ISO/TS 11405:2015; Dentistry—Testing of Adhesion to Tooth Structure. ISO International Organization for Standardization: Geneva, Switzerland, 2015.
- Bizhang, M.; Schmidt, I.; Chun, Y.-H.P.; Arnold, W.H.; Zimmer, S. Toothbrush Abrasivity in a Long-Term Simulation on Human Dentin Depends on Brushing Mode and Bristle Arrangement. PLoS ONE 2017, 12, e0172060. [Google Scholar] [CrossRef]
- ISO 4287:1997; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions, and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 1997.
- Daniele, V.; Macera, L.; Taglieri, G.; Di Giambattista, A.; Spagnoli, G.; Massaria, A.; Messori, M.; Quagliarini, E.; Chiappini, G.; Campanella, V.; et al. Thermoplastic discs used for commercial orthodontic aligners: Complete physicochemical and mechanical characterization. Materials 2020, 13, 2386. [Google Scholar] [CrossRef]
- Babanouri, N.; Ahmadi, N.; Pakshir, H.R.; Ajami, S.; Habibagahi, R. Influence of a bleaching agent on surface and mechanical properties of orthodontic thermoplastic retainer materials: An in vitro study. J. Orofac. Orthop. 2022, 83, 332–338. [Google Scholar] [CrossRef]
- Goiato, J.C.V.; Lopes, V.T.; de Moraes Melo Neto, C.L.; de Magalhães Bertoz, A.P.; dos Santos, D.M.; Bento, V.A.A.; Goiato, M.C. Effect of Extrinsic Pigmentation on Dimensional Stability, Hardness, Detail Reproduction and Color of a Silicone. Eur. J. Dent. 2022, 1, 1–260. [Google Scholar] [CrossRef] [PubMed]
- Ryokawa, H.; Miyazaki, Y.; Fujishima, A.; Miyazaki, T.; Maki, K. The mechanical properties of dental thermoplastic materials in a simulated intraoral environment. Orthod. Waves. 2006, 65, 64–72. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Alwafi, A.; Liu, X.; Andrews, J.; Ludwig, B.; Bichu, A.Y.; Zou, B. Advances in orthodontic clear aligner materials. Bioact. Mater. 2022, 22, 384–403. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Kohda, N.; Kawaguchi, K.; Muguruma, T.; Ohta, M.; Naganishi, A.; Murakami, T.; Mizoguchi, I. Effects of temperature changes and stress loading on the mechanical and shape memory properties of thermoplastic materials with different glass transition behaviors and crystal structures. Eur. J. Orthod. 2015, 37, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Dalaie, K.; Fatemi, S.M.; Ghaffari, S. Dynamic mechanical and thermal properties of clear aligners after thermoforming and aging. Prog. Orthod. 2021, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Bucci, R.; Rongo, R.; Levatè, C.; Michelotti, A.; Barone, S.; Razionale, A.V.; D’Antò, V. Thickness of orthodontic clear aligners after thermoforming and after 10 days of intraoral exposure: A prospective clinical study. Prog. Orthod. 2019, 20, 36. [Google Scholar] [CrossRef]
- Moreno Nieto, D.; Alonso-García, M.; Pardo-Vicente, M.-A.; Rodríguez-Parada, L. Product Design by Additive Manufacturing for Water Environments: Study of Degradation and Absorption Behavior of PLA and PETG. Polymers 2021, 13, 1036. [Google Scholar] [CrossRef]
- Zhang, N.; Bai, Y.; Ding, X.; Zhang, Y. Preparation and characterization of thermoplastic materials for invisible orthodontics. Dent. Mater. J. 2011, 30, 954–959. [Google Scholar] [CrossRef]
- Peracini, A.; Andrade, I.M.; de Paranhos, H.d.F.O.; da Silva, C.H.L.; de Souza, R.F. Behaviors and hygiene habits of complete denture wearers. Braz. Dent. J. 2010, 21, 247–252. [Google Scholar] [CrossRef]
- Chang, C.S.; Al-Awadi, S.; Ready, D.; Noar, J. An assessment of the effectiveness of mechanical and chemical cleaning of Essix orthodontic retainer. J. Orthod. 2014, 41, 110–117. [Google Scholar] [CrossRef]
- Niemi, M.-L.; Sandholm, L.; Ainamo, J. Frequency of gingival lesions after standardized brushing as related to stiffness of toothbrush and abrasiveness of dentifrice. J. Clin. Periodontol. 1984, 11, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Suter, F.; Zinelis, S.; Patcas, R.; Schätzle, M.; Eliades, G.; Eliades, T. Roughness and wettability of aligner materials. J. Orthod. 2020, 47, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Sarret, D.C. Polishing systems. ADA Prof. Product Rev. 2010, 5, 1–16. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimunović, L.; Blagec, T.; Meštrović, S. Resistance of PETG Materials on Thermocycling and Brushing. Dent. J. 2023, 11, 135. https://doi.org/10.3390/dj11050135
Šimunović L, Blagec T, Meštrović S. Resistance of PETG Materials on Thermocycling and Brushing. Dentistry Journal. 2023; 11(5):135. https://doi.org/10.3390/dj11050135
Chicago/Turabian StyleŠimunović, Luka, Tadeja Blagec, and Senka Meštrović. 2023. "Resistance of PETG Materials on Thermocycling and Brushing" Dentistry Journal 11, no. 5: 135. https://doi.org/10.3390/dj11050135
APA StyleŠimunović, L., Blagec, T., & Meštrović, S. (2023). Resistance of PETG Materials on Thermocycling and Brushing. Dentistry Journal, 11(5), 135. https://doi.org/10.3390/dj11050135