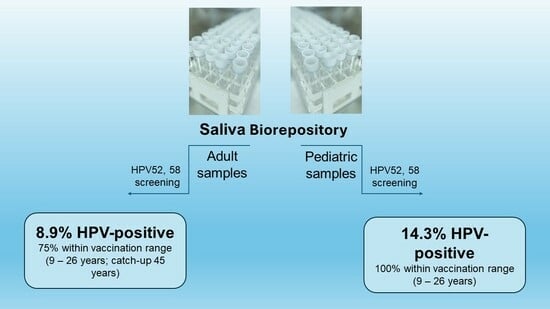
Screening for High-Risk Human Papillomavirus Reveals HPV52 and HPV58 among Pediatric and Adult Patient Saliva Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Approval
2.2. Original Sample Collection Protocol
2.3. Sample Processing and Analysis
2.4. Quantitative Polymerase Chain Reaction (qPCR) Screening
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Ni, Z.; Wei, T.; Liu, Q. Persistent HPV infection after conization of cervical intraepithelial neoplasia—A systematic review and meta-analysis. BMC Women’s Health 2023, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Volesky, K.D.; El-Zein, M.; Franco, E.L.; Brenner, D.R.; Friedenreich, C.M.; Ruan, Y.; ComPARe Study Team. Cancers attributable to infections in Canada. Prev. Med. 2019, 122, 109–117, Erratum in Prev. Med. 2019, 129, 105730. [Google Scholar] [CrossRef]
- De Oliveira, G.G.; Gonçalves, A.K.; Eleutério, J.; Pinheiro, L.G.P. Systematic review and meta-analysis of the papillomavirus prevalence in breast cancer fresh tissues. Breast Dis. 2021, 41, 123–132. [Google Scholar] [CrossRef]
- Karnosky, J.; Dietmaier, W.; Knuettel, H.; Freigang, V.; Koch, M.; Koll, F.; Zeman, F.; Schulz, C. HPV and lung cancer: A systematic review and meta-analysis. Cancer Rep. 2021, 4, e1350. [Google Scholar] [CrossRef]
- Jakobsen, K.K.; Carlander, A.-L.F.; Bendtsen, S.K.; Garset-Zamani, M.; Lynggaard, C.D.; Grønhøj, C.; von Buchwald, C. Diagnostic Accuracy of HPV Detection in Patients with Oropharyngeal Squamous Cell Carcinomas: A Systematic Review and Meta-Analysis. Viruses 2021, 13, 1692. [Google Scholar] [CrossRef]
- De la Cour, C.D.; Sperling, C.D.; Belmonte, F.; Syrjänen, S.; Verdoodt, F.; Kjaer, S.K. Prevalence of human papillomavirus in oral epithelial dysplasia: Systematic review and meta-analysis. Head Neck 2020, 42, 2975–2984. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, P.; Fani, M.; Rezayi, M.; Avan, A.; Pasdar, Z.; Karimi, E.; Amiri, I.S.; Ghayour-Mobarhan, M. Early detection of cervical cancer based on high-risk HPV DNA-based genosensors: A systematic review. BioFactors 2019, 45, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Álvarez, M.I.; Gómez-Urquiza, J.L.; Ahmed, H.H.-E.; Albendín-García, L.; Gómez-Salgado, J.; la Fuente, G.A.C.-D. Prevalence and Risk Factors of Human Papillomavirus in Male Patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2210. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.; De Maria, D.; Francavilla, A.; Di Gaetano, C.; Ronco, G.; Naccarati, A. MicroRNAs as markers of progression in cervical cancer: A systematic review. BMC Cancer 2018, 18, 696. [Google Scholar] [CrossRef]
- Pelizzer, T.; Dias, C.P.; Poeta, J.; Torriani, T.; Roncada, C. Prevalência de câncer colorretal associado ao papilomavírus humano: Uma revisão sistemática com metanálise. Rev. Bras. Epidemiol. 2016, 19, 791–802. [Google Scholar] [CrossRef]
- Kansy, K.; Thiele, O.; Freier, K. The role of human papillomavirus in oral squamous cell carcinoma: Myth and reality. Oral Maxillofac. Surg. 2014, 18, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rosalik, K.; Tarney, C.; Han, J. Human Papilloma Virus Vaccination. Viruses 2021, 13, 1091. [Google Scholar] [CrossRef]
- Olsson, S.-E.; Restrepo, J.A.; Reina, J.C.; Pitisuttithum, P.; Ulied, A.; Varman, M.; Van Damme, P.; Moreira, E.D., Jr.; Ferris, D.; Block, S.; et al. Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in girls and boys 9 to 15 years of age: Interim analysis after 8 years of follow-up. Papillomavirus Res. 2020, 10, 100203. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Joura, E.A.; Garland, S.M.; Huh, W.K.; Iversen, O.-E.; Kjaer, S.K.; Ferenczy, A.; Kurman, R.J.; Ronnett, B.M.; Stoler, M.H.; et al. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: Comparison with historic placebo population. Gynecol. Oncol. 2019, 154, 110–117. [Google Scholar] [CrossRef]
- Pils, S.; Joura, E. From the monovalent to the nine-valent HPV vaccine. Clin. Microbiol. Infect. 2015, 21, 827–833. [Google Scholar] [CrossRef]
- Wang, D.; Liu, X.; Wei, M.; Qian, C.; Song, S.; Chen, J.; Wang, Z.; Xu, Q.; Yang, Y.; He, M.; et al. Rational design of a multi-valent human papillomavirus vaccine by capsomere-hybrid co-assembly of virus-like particles. Nat. Commun. 2020, 11, 2841. [Google Scholar] [CrossRef]
- Dochez, C.; Bogers, J.J.; Verhelst, R.; Rees, H. HPV vaccines to prevent cervical cancer and genital warts: An update. Vaccine 2014, 32, 1595–1601. [Google Scholar] [CrossRef]
- Cody, P.; Tobe, K.; Abe, M.; Elbasha, E.H. Public health impact and cost effectiveness of routine and catch-up vaccination of girls and women with a nine-valent HPV vaccine in Japan: A model-based study. BMC Infect. Dis. 2021, 21, 11. [Google Scholar] [CrossRef]
- Paz-Zulueta, M.; Alvarez-Paredes, L.; Rodriguez Diaz, J.C.; Paras-Bravo, P.; Andrada Becerra, M.E.; Rodriguez Ingelmo, J.M.; Ruiz Garcia, M.M.; Portilla, J.; Santibanez, M. Prevalence of high-risk HPV genotypes, categorised by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC Cancer 2018, 18, 112. [Google Scholar] [CrossRef]
- Rahman, S.; Giuliano, A.R.; Rollison, D.E.; Pawlita, M.; Waterboer, T.; Villa, L.L.; Ponce, E.L. Cutaneous HPV and alpha-mucosal 9-valent HPV sero-status associations. Papillomavirus Res. 2017, 4, 54–57. [Google Scholar] [CrossRef]
- Signorelli, C.; Odone, A.; Ciorba, V.; Cella, P.; Audisio, R.A.; Lombardi, A.; Mariani, L.; Mennini, F.S.; Pecorelli, S.; Rezza, G.; et al. Human papillomavirus 9-valent vaccine for cancer prevention: A systematic review of the available evidence. Epidemiol. Infect. 2017, 145, 1962–1982. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, M.; Videla, S.; Darwich, L.; Tarrats, A.; Piñol, M.; García-Cuyás, F.; Llatjos, M.; Alcalde, C.; Fernández, I.; Sirera, G.; et al. Human papillomavirus HPV-16, 18, 52 and 58 integration in cervical cells of HIV-1-infected women. J. Clin. Virol. 2010, 48, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Yang, Z.; Li, X.; Chen, M.; Liu, J.; Zhang, Y.; Sun, X. HPV-16, HPV-58, and HPV-33 are the most carcinogenic HPV genotypes in Southwestern China and their viral loads are associated with severity of premalignant lesions in the cervix. Virol. J. 2018, 15, 94. [Google Scholar] [CrossRef]
- Kukimoto, I.; Onuki, M.; Yamamoto, K.; Yahata, H.; Aoki, Y.; Yokota, H.; Konnai, K.; Nio, A.; Takehara, K.; Kamiura, S.; et al. Regional differences in human papillomavirus type 52 prevalence among Japanese women with cervical intraepithelial neoplasia. Ultrasound Med. Biol. 2022, 52, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ma, Y.-Y.; Moh, J.-S.; Ou, Y.-C.; Shen, S.-Y.; ChangChien, C.-C. High prevalence of genital human papillomavirus type 52 and 58 infection in women attending gynecologic practitioners in South Taiwan. Gynecol. Oncol. 2006, 101, 40–45. [Google Scholar] [CrossRef]
- Kim, T.E.; Kim, H.W.; Lee, K.E. Distribution of Human Papillomavirus 52 and 58 Genotypes, and Their Expression of p16 and p53 in Cervical Neoplasia. Korean J. Pathol. 2014, 48, 24–29. [Google Scholar] [CrossRef]
- De Oliveira, B.R.; e Silva, B.V.D.; dos Santos, K.C.; Caetano, K.A.A.; Mota, G.; Saddi, V.A.; Rabelo-Santos, S.H.; Villa, L.L.; Vaddiparti, K.P.; Cook, R.L.; et al. Human Papillomavirus Positivity at 3 Anatomical Sites Among Transgender Women in Central Brazil. Sex. Transm. Dis. 2023, 50, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Kornhaber, M.S.; Florence, T.; Davis, T.; Kingsley, K. Assessment of Oral Human Papillomavirus Prevalence in Pediatric and Adult Patients within a Multi-Ethnic Clinic Population. Dent. J. 2022, 10, 54. [Google Scholar] [CrossRef]
- Hinton, H.; Coleman, S.; Salem, J.R.; Kingsley, K. Screening for High-Risk Oral Human Papillomavirus (HPV31, HPV33, HPV35) in a Multi-Racial Pediatric and Adult Clinic Patient Population. Cancers 2023, 15, 4501. [Google Scholar] [CrossRef]
- Nunes, L.A.S.; Mussavira, S.; Bindhu, O.S. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem. Medica 2015, 25, 177–192. [Google Scholar] [CrossRef]
- Ilea, A.; Andrei, V.; Feurdean, C.N.; Băbțan, A.-M.; Petrescu, N.B.; Câmpian, R.S.; Boșca, A.B.; Ciui, B.; Tertiș, M.; Săndulescu, R.; et al. Saliva, a Magic Biofluid Available for Multilevel Assessment and a Mirror of General Health—A Systematic Review. Biosensors 2019, 9, 27. [Google Scholar] [CrossRef]
- Ibrahimi, N.; Delaunay-Moisan, A.; Hill, C.; Le Teuff, G.; Rupprecht, J.-F.; Thuret, J.-Y.; Chaltiel, D.; Potier, M.-C. Screening for SARS-CoV-2 by RT-PCR: Saliva or nasopharyngeal swab? Rapid review and meta-analysis. PLoS ONE 2021, 16, e0253007. [Google Scholar] [CrossRef]
- Auguste, A.; Gaëte, S.; Herrmann-Storck, C.; Michineau, L.; Joachim, C.; Deloumeaux, J.; Duflo, S.; Luce, D. Prevalence of oral HPV infection among healthy individuals and head and neck cancer cases in the French West Indies. Cancer Causes Control. 2017, 28, 1333–1340. [Google Scholar] [CrossRef]
- Muzio, L.L.; Ballini, A.; Cantore, S.; Bottalico, L.; Charitos, I.A.; Ambrosino, M.; Nocini, R.; Malcangi, A.; Dioguardi, M.; Cazzolla, A.P.; et al. Overview of Candida albicans and Human Papillomavirus (HPV) Infection Agents and their Biomolecular Mechanisms in Promoting Oral Cancer in Pediatric Patients. BioMed Res. Int. 2021, 2021, 7312611. [Google Scholar] [CrossRef]
- Di Spirito, F.; Pantaleo, G.; Di Palo, M.P.; Amato, A.; Raimondo, A.; Amato, M. Oral Human Papillomavirus Benign Lesions and HPV-Related Cancer in Healthy Children: A Systematic Review. Cancers 2023, 15, 1096. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Kumar, L.; Dhasmana, A.; Mitra, T.; Dey, A.; Malik, S.; Kim, B.; Gundamaraju, R. Governing HPV-related carcinoma using vaccines: Bottlenecks and breakthroughs. Front. Oncol. 2022, 12, 977933. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.; Cheng, M.A.; Hung, C.F.; Wu, T.C. Vaccination Strategies for the Control and Treatment of HPV Infection and HPV-Associated Cancer. Recent Results Cancer Res. 2021, 217, 157–195. [Google Scholar] [CrossRef] [PubMed]
- Morales-Campos, D.Y.; Zimet, G.D.; Kahn, J.A. Human Papillomavirus Vaccine Hesitancy in the United States. Pediatr. Clin. N. Am. 2023, 70, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, S.; Fang, Y. Parental Willingness and Associated Factors of Pediatric Vaccination in the Era of COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1453. [Google Scholar] [CrossRef]
- Dempsey, A.F.; O’Leary, S.T. Human Papillomavirus Vaccination: Narrative Review of Studies on How Providers’ Vaccine Communication Affects Attitudes and Uptake. Acad. Pediatr. 2018, 18, S23–S27. [Google Scholar] [CrossRef] [PubMed]
- Achimaș-Cadariu, T.; Pașca, A.; Jiboc, N.-M.; Puia, A.; Dumitrașcu, D.L. Vaccine Hesitancy among European Parents—Psychological and Social Factors Influencing the Decision to Vaccinate against HPV: A Systematic Review and Meta-Analysis. Vaccines 2024, 12, 127. [Google Scholar] [CrossRef]
- Chan, D.N.; Li, C.; Law, B.M.; Choi, K.; Lee, P.P.; So, W.K. Factors affecting HPV vaccine uptake among ethnic minority adolescent girls: A systematic review and meta-analysis. Asia-Pac. J. Oncol. Nurs. 2023, 10, 100279. [Google Scholar] [CrossRef]
- Vollrath, K.; Thul, S.; Holcombe, J. Meaningful Methods for Increasing Human Papillomavirus Vaccination Rates: An Integrative Literature Review. J. Pediatr. Health Care 2018, 32, 119–132. [Google Scholar] [CrossRef]
- Bratic, J.S.; Seyferth, E.R.; Bocchini, J.A. Update on barriers to human papillomavirus vaccination and effective strategies to promote vaccine acceptance. Curr. Opin. Pediatr. 2016, 28, 407–412. [Google Scholar] [CrossRef]
- Gilkey, M.B.; McRee, A.-L. Provider communication about HPV vaccination: A systematic review. Hum. Vaccines Immunother. 2016, 12, 1454–1468. [Google Scholar] [CrossRef]
- De Souza, M.M.A.; Hartel, G.; Olsen, C.M.; Whiteman, D.C.; Antonsson, A. Oral human papillomavirus (HPV) infection and HPV vaccination in an Australian cohort. Int. J. Cancer 2023, 153, 417–426. [Google Scholar] [CrossRef]
- Vasani, S.; Frazer, I.; Punyadeera, C. Determining the utility of a screening program to reduce the incidence of HPV driven oropharyngeal cancer. Oncoscience 2021, 8, 91–93. [Google Scholar] [CrossRef]
- Sharma, S.; Kumari, B.; Ali, A.; Sharma, A.K.; Gehlot, A. Non-invasive saliva-based screening of high-risk Human Papilloma Virus 16 and 18 in healthy young adults and creating awareness about its vaccination. J. Fam. Med. Prim. Care 2021, 10, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.O.; Jones, K.; Naavaal, S.; O’Connell, J.M.; Dds, C.D.; Arlotta, D. Estimating the cost of school sealant programs with minimal data. J. Public Health Dent. 2018, 78, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.C.; Zhou, W.; McCoy, S.J.; McDonough, I.K.; Burston, B.; Ditmyer, M.; Shen, J.J. Factors Associated with Preventable Emergency Department Visits for Nontraumatic Dental Conditions in the U.S. Int. J. Environ. Res. Public Health 2019, 16, 3671. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Tewari, S.R.; Troy, T.; Webster-Cyriaque, J.; Wiley, D.J.; Lahiri, C.D.; Palella, F.J.; Gillison, M.L.; Strickler, H.D.; Struijk, L.; et al. Oncogenic Oral Human Papillomavirus Clearance Patterns over 10 Years. Cancer Epidemiol. Biomark. Prev. 2024, OF1–OF9. [Google Scholar] [CrossRef] [PubMed]
- D’souza, G.; Clemens, G.; Strickler, H.D.; Wiley, D.J.; Troy, T.; Struijk, L.; Gillison, M.; Fakhry, C. Long-term Persistence of Oral HPV Over 7 Years of Follow-up. JNCI Cancer Spectr. 2020, 4, pkaa047. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.O.; Sugar, E.A.; Cranston, R.D.; Weber, K.M.; Burk, R.D.; Wiley, D.J.; Reddy, S.; Margolick, J.B.; Strickler, H.D.; Wentz, A.; et al. The association of medication use with clearance or persistence of oral HPV infection. Cancer Causes Control. 2016, 27, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- O’connor, E.A.; Perdue, L.A.; Coppola, E.L.; Henninger, M.L.; Thomas, R.G.; Gaynes, B.N. Depression and Suicide Risk Screening: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2023, 329, 2068–2085. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Bessonova, L.; Hughes, R.; Doane, M.J.; O’sullivan, A.K.; Snook, K.; Cichewicz, A.; Weiden, P.J.; Harvey, P.D. Systematic Review of Real-World Treatment Patterns of Oral Antipsychotics and Associated Economic Burden in Patients with Schizophrenia in the United States. Adv. Ther. 2022, 39, 3933–3956. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.; Joachim, C.; Deloumeaux, J.; Gaete, S.; Michineau, L.; Herrmann-Storck, C.; Duflo, S.; Luce, D. Head and neck cancer risk factors in the French West Indies. BMC Cancer. 2021, 21, 1071. [Google Scholar] [CrossRef]
- Ho, W.C.S.; Boon, S.S.; Chong, K.C.; Lai, C.K.C.; Sze, R.K.H.; Khan, A.T.K.; Xing, R.L.; Sukarom, I.; Wu, Y.; Chau, R.W.Y.; et al. Prevalence of oral human papillomavirus infection among the general adult population in Hong Kong. J. Med Virol. 2024, 96, e29460. [Google Scholar] [CrossRef]
- Nielsen, K.J.; Jakobsen, K.K.; Jensen, J.S.; Grønhøj, C.; Von Buchwald, C. The Effect of Prophylactic HPV Vaccines on Oral and Oropharyngeal HPV Infection—A Systematic Review. Viruses 2021, 13, 1339. [Google Scholar] [CrossRef]
- Berman, T.A.; Schiller, J.T. Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer. 2017, 123, 2219–2229. [Google Scholar] [CrossRef]
- Beachler, D.C.; Kreimer, A.R.; Schiffman, M.; Herrero, R.; Wacholder, S.; Rodriguez, A.C.; Lowy, D.R.; Porras, C.; Schiller, J.T.; Quint, W.; et al. Multisite HPV16/18 Vaccine Efficacy Against Cervical, Anal, and Oral HPV Infection. JNCI J. Natl. Cancer Inst. 2015, 108, djv302. [Google Scholar] [CrossRef]
- Alqahtani, W.S.; Almufareh, N.A.; Al-Johani, H.A.; Alotaibi, R.K.; Juliana, C.I.; Aljarba, N.H.; Alqahtani, A.S.; Almarshedy, B.; Elasbali, A.M.; Ahmed, H.G.; et al. Oral and Oropharyngeal Cancers and Possible Risk Factors Across Gulf Cooperation Council Countries: A Systematic Review. World J. Oncol. 2020, 11, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Chetwood, J.D.; Garg, P.; Finch, P.; Gordon, M. Systematic review: The etiology of esophageal squamous cell carcinoma in low-income settings. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Jin, H.; Fu, X. Comparative efficacy of human papillomavirus vaccines: Systematic review and network meta-analysis. Expert Rev. Vaccines 2023, 22, 1168–1178. [Google Scholar] [CrossRef] [PubMed]


| Demographic of Adult Patient Samples | Study Samples | UNLV-SDM Clinic | Statistical Analysis |
|---|---|---|---|
| Sex (Adult) | |||
| Adult Males | 44.4% (n = 20/45) | 49.1% (Adult clinic) | X2 = 1.000, d.f. = 1 |
| Adult Females | 55.6% (n = 25/45) | 50.9% (Adult clinic) | p = 0.3172 |
| Ethnicity/Race (Adult) | |||
| White/Caucasian | 31.1% (n = 14/45) | 34.6% (Adult clinic) | X2 = 0.713, d.f. = 1 |
| Minority | 68.9% (n = 31/45) | 65.4% (Adult clinic) | p = 0.4017 |
| Hispanic | 57.8% (n = 26/45) | 58.6% (Adult clinic) | |
| Age (Adult) | |||
| Average Range | 41.2 years 18–81 years |
42.3 years 18–89 years |
Two-tailed t-test p = 0.711 |
| Demographic Variable | HPV-Negative (n = 41/45) | HPV-Positive (n = 4/45) | Statistical Analysis: Fisher’s Exact Test |
|---|---|---|---|
| Males (Adult) | 43.9% (n = 18/41) | 50% (n = 2/4) | |
| Females (Adult) | 56.1% (n = 23/41) | 50% (n = 2/4) | p = 0.8418 |
| Total | 91.1% (n = 41/45) | 8.9% (n = 4/45) | |
| Non-Minority (Adult) | 29.3% (n = 12/41) | 25% (n = 1/4) | |
| Minority (Adult) | 70.7% (n = 29/41) | 75% (n = 3/4) | p = 0.8573 |
| Total | 91.1% (n = 41/45) | 8.9% (n = 4/45) | |
| Vaccination range | 63.4% (n = 26/41) | 75% (n = 3/4) | |
| Non-vaccination range | 36.6% (n = 15/41) | 25% (n = 1/4) | p = 0.7869 |
| Average age | 43.8 years | 41.3 years |
| Demographic | Study Samples | UNLV-SDM Clinic | Statistical Analysis |
|---|---|---|---|
| Sex (Pediatric) | |||
| Males (Pediatric) | 42.9% (n = 18/42) | 47.2% (Pediatric clinic) | X2 = 0.642, d.f. = 1 |
| Females (Pediatric) | 57.1% (n = 24/42) | 52.8% (Pediatric clinic) | p = 0.4229 |
| Ethnicity/Race (Pediatric) | |||
| White/Caucasian | 16.7% (n = 7/42) | 24.7% (Pediatric clinic) | X2 = 3.413, d.f. = 1 |
| Minority | 83.3% (n = 35/42) | 75.3% (Pediatric clinic) | p = 0.0647 |
| Hispanic | 54.7% (n = 23/42) | 52.1% (Pediatric clinic) | |
| Age (Pediatric) | |||
|
Average Range |
12.3 years 5–17 years |
10.4 years 0–17 years |
Two-tailed t-test p = 0.2331 |
| Demographic Variable | HPV-Negative (n = 36/42) | HPV-Positive (n = 6/42) | Statistical Analysis: Fisher’s Exact Test |
|---|---|---|---|
| Males (Pediatric) | 44.4% (n = 16/36) | 33.3% (n = 2/6) | |
| Females (Pediatric) | 55.6% (n = 20/36) | 66.7% (n = 4/6) | p = 0.6852 |
| Total | 85.7% (n = 36/42) | 14.3% (n = 6/42) | |
| non-Minority (Pediatric) | 19.4% (n = 7/36) | 33.3% (n = 2/6) | |
| Minority (Pediatric) | 80.6% (n = 29/36) | 66.7% (n = 4/6) | p = 0.5928 |
| Total | 85.7% (n = 36/42) | 14.3% (n = 6/42) | |
| Vaccination range | 66.7% (n = 24/36) | 100% (n = 6/6) | X2 = 49.254, d.f. = 1 |
| Non-vaccination range | 33.3% (n = 12/36) | 0% (n = 0/6) | p = 0.1589 |
| Average age | 12.3 years | 12.8 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinton, H.; Herrera, L.; Valenzuela, S.; Howard, K.M.; Kingsley, K. Screening for High-Risk Human Papillomavirus Reveals HPV52 and HPV58 among Pediatric and Adult Patient Saliva Samples. Dent. J. 2024, 12, 56. https://doi.org/10.3390/dj12030056
Hinton H, Herrera L, Valenzuela S, Howard KM, Kingsley K. Screening for High-Risk Human Papillomavirus Reveals HPV52 and HPV58 among Pediatric and Adult Patient Saliva Samples. Dentistry Journal. 2024; 12(3):56. https://doi.org/10.3390/dj12030056
Chicago/Turabian StyleHinton, Hunter, Lorena Herrera, Sofia Valenzuela, Katherine M. Howard, and Karl Kingsley. 2024. "Screening for High-Risk Human Papillomavirus Reveals HPV52 and HPV58 among Pediatric and Adult Patient Saliva Samples" Dentistry Journal 12, no. 3: 56. https://doi.org/10.3390/dj12030056
APA StyleHinton, H., Herrera, L., Valenzuela, S., Howard, K. M., & Kingsley, K. (2024). Screening for High-Risk Human Papillomavirus Reveals HPV52 and HPV58 among Pediatric and Adult Patient Saliva Samples. Dentistry Journal, 12(3), 56. https://doi.org/10.3390/dj12030056