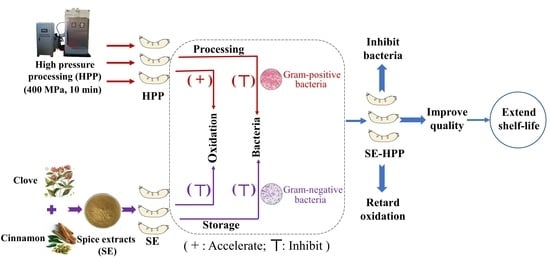
Combined Effect of High-Pressure Processing with Spice Extracts on Quality of Low-Salt Sausage during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Spice Extracts
2.2. Preparation of Low-Salt Sausage
2.3. Measurement of pH Value
2.4. Color Measurement
2.5. Determination of Thiobarbituric Acid Reactive Substances (TBARS) Value
2.6. Measurement of Carbonyl Content
2.7. Microbiological Analysis
2.7.1. Microbial Counts
2.7.2. High-Throughput Sequencing
2.8. Statistical Analysis
3. Results and Discussion
3.1. pH Value
3.2. Color
3.3. TBARS Values
3.4. Carbonyl Content
3.5. Microbial Analysis
3.5.1. Microbial Community Using the Culture-Dependent Method
3.5.2. Microbial Diversity Using High-Throughput Sequencing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quilaqueo, M.; Duizer, L.; Aguilera, J.M. The morphology of salt crystals affects the perception of saltiness. Food Res. Int. 2015, 76, 675–681. [Google Scholar] [CrossRef]
- Freitag, A.; Cluff, M.; Hitzeroth, A.C.; van Wyngaardt, L.; Hugo, A.; Hugo, C.J. Community level physiological profiling of reduced or replaced salt fresh sausage inoculated with Escherichia coli ATCC 25922. LWT-Food Sci. Technol. 2021, 148, 111786. [Google Scholar] [CrossRef]
- Tamm, A.; Bolumar, T.; Bajovic, B.; Toepfl, S. Salt (NaCl) reduction in cooked ham by a combined approach of high pressure treatment and the salt replacer KCl. Innov. Food Sci. Emerg. Technol. 2016, 36, 294–302. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Zhu, J.; Kong, B.; Liu, Q.; Chen, Q. Improving the taste profile of reduced-salt dry sausage by inoculating different lactic acid bacteria. Food Res. Int. 2021, 145, 110391. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; Astray, G.; Gullón, B.; Franco, D.; Campagnol, P.C.B.; Lorenzo, J.M. Inclusion of seaweeds as healthy approach to formulate new low-salt meat products. Curr. Opin. Food Sci. 2020, 40, 20–25. [Google Scholar] [CrossRef]
- Pinton, M.B.; dos Santos, B.A.; Lorenzo, J.M.; Cichoski, A.J.; Boeira, C.P.; Campagnol, P.C.B. Green technologies as a strategy to reduce NaCl and phosphate in meat products: An overview. Curr. Opin. Food Sci. 2020, 40, 1–5. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Pietrasik, Z.; Gaudette, N.; Johnston, S. The use of high pressure processing to enhance the quality and shelf life of reduced sodium naturally cured restructured cooked hams. Meat Sci. 2016, 116, 102–109. [Google Scholar] [CrossRef]
- Bampi, M.; Domschke, N.; Schmidt, F.; Laurindo, J. Influence of vacuum application, acid addition and partial replacement of NaCl by KCl on the mass transfer during salting of beef cuts. LWT-Food Sci. Technol. 2016, 74, 26–33. [Google Scholar] [CrossRef]
- Bidlas, E.; Lambert, R.J. Comparing the antimicrobial effectiveness of NaCl and KCl with a view to salt/sodium replacement. Int. J. Food Microbiol. 2008, 124, 98–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Yan, W.; Zhuang, H.; Huang, M.; Zhao, J.; Zhang, J. Oxidative stability and antioxidant enzyme activities of dry-cured bacons as affected by the partial substitution of NaCl with KCl. Food Chem. 2016, 201, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Huang, A.; Fricke, B.C.; Schadler, K.L.; Parker, N.H. Preliminary Evidence on the Effects of Exercise on Tumor Biology: A Potential Guide for Prescribing Exercise. Curr. Phys. Med. Rehabilitation Rep. 2021, 9, 136–141. [Google Scholar] [CrossRef]
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K.H.; Guyon, C.; Stübler, A.; De Lamballerie, M.; Hertel, C.; Brüggemann, D.A. High-pressure processing of meat: Molecular impacts and industrial applications. Compr. Rev. Food Sci. Food Saf. 2020, 20, 332–368. [Google Scholar] [CrossRef]
- Hygreeva, D.; Pandey, M.C. Novel approaches in improving the quality and safety aspects of processed meat products through high pressure processing technology—A review. Trends Food Sci. Technol. 2016, 54, 175–185. [Google Scholar] [CrossRef]
- O’Flynn, C.C.; Cruz-Romero, M.C.; Troy, D.; Mullen, A.M.; Kerry, J.P. The application of high-pressure treatment in the reduction of salt levels in reduced-phosphate breakfast sausages. Meat Sci. 2014, 96, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, Y.; Zhao, L.; Wang, Y.; Rao, L.; Liao, X. Pressure-resistant acclimation of lactic acid bacteria from a natural fermentation production product using high pressure. Innov. Food Sci. Emerg. Technol. 2021, 69, 102660. [Google Scholar] [CrossRef]
- Clariana, M.; Guerrero, L.; Sárraga, C.; Díaz, I.; Valero, Á.; García-Regueiro, J.A. Influence of high pressure application on the nutritional, sensory and microbiological characteristics of sliced skin vacuum packed dry-cured ham. Effects along the storage period. Innov. Food Sci. Emerg. Technol. 2011, 12, 456–465. [Google Scholar] [CrossRef]
- Kalchayanand, N.; Sikes, A.; Dunne, C.; Ray, B. Factors influencing death and injury of foodborne pathogens by hydrostatic pressure-pasteurization. Food Microbiol. 1998, 15, 207–214. [Google Scholar] [CrossRef]
- Guyon, C.; Meynier, A.; de Lamballerie, M. Protein and lipid oxidation in meat: A review with emphasis on high-pressure treatments. Trends Food Sci. Technol. 2016, 50, 131–143. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barnaba, C.; Barbosa-Cánovas, G.V. Effects of high pressure processing on lipid oxidation: A review. Innov. Food Sci. Emerg. Technol. 2014, 22, 1–10. [Google Scholar] [CrossRef]
- Kantono, K.; Hamid, N.; Oey, I.; Wu, Y.C.; Ma, Q.; Farouk, M.; Chanda, D. Effect of High Hydrostatic Pressure Processing on the Chemical Characteristics of Different Lamb Cuts. Foods 2020, 9, 1444. [Google Scholar] [CrossRef]
- Chen, H. Temperature-assisted pressure inactivation of Listeria monocytogenes in Turkey breast meat. Int. J. Food Microbiol. 2007, 117, 55–60. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Guo, X. Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci. Hum. Wellness 2016, 5, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Pateiro, M.; Munekata, P.E.; Cittadini, A.; Domínguez, R.; Lorenzo, J.M. Metallic-based salt substitutes to reduce sodium content in meat products. Curr. Opin. Food Sci. 2020, 38, 21–31. [Google Scholar] [CrossRef]
- Weerakkody, N.; Caffin, N.; Turner, M.; Dykes, G.A. In vitro antimicrobial activity of less-utilized spice and herb extracts against selected food-borne bacteria. Food Control 2010, 21, 1408–1414. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, X.; Chen, H.; Zhang, C.; Kong, B. Impact of spice extracts on the formation of biogenic amines and the physicochemical, microbiological and sensory quality of dry sausage. Food Control 2018, 92, 190–200. [Google Scholar] [CrossRef]
- Basak, S.; Singh, J.K.; Morri, S.; Shetty, P.H. Assessment and modelling the antibacterial efficacy of vapours of cassia and clove essential oils against pathogens causing foodborne illness. LWT-Food Sci. Technol. 2021, 150, 112076. [Google Scholar] [CrossRef]
- Chuesiang, P.; Sanguandeekul, R.; Siripatrawan, U. Phase inversion temperature-fabricated cinnamon oil nanoemulsion as a natural preservative for prolonging shelf-life of chilled Asian seabass (Lates calcarifer) fillets. LWT-Food Sci. Technol. 2020, 125, 109122. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Li, D.; Zhang, W.; Jiang, M.; Chen, X.H.; Dong, M.S. Comparative analysis of the bacterial diversity of Chinese fermented sausages using high-throughput sequencing. LWT-Food Sci. Technol. 2021, 150, 111975. [Google Scholar] [CrossRef]
- Liu, D.Y.; Xiao, X.; Wang, H.H.; Zhang, Q.Y.; Zou, Y.F. Characterization of the bacterial community of braised chicken, a specialty poultry product in China. Poult. Sci. 2019, 98, 1055–1063. [Google Scholar] [CrossRef]
- Li, P.; Kong, B.; Chen, Q.; Zheng, D.; Liu, N. Formation and identification of nitrosylmyoglobin by Staphylococcus xylosus in raw meat batters: A potential solution for nitrite substitution in meat products. Meat Sci. 2012, 93, 67–72. [Google Scholar] [CrossRef]
- Jayawardana, B.; Liyanage, R.; Lalantha, N.; Iddamalgoda, S.; Weththasinghe, P. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT-Food Sci. Technol. 2015, 64, 1204–1208. [Google Scholar] [CrossRef]
- Berardo, A.; De Maere, H.; Stavropoulou, D.; Rysman, T.; Leroy, F.; De Smet, S. Effect of sodium ascorbate and sodium nitrite on protein and lipid oxidation in dry fermented sausages. Meat Sci. 2016, 121, 359–364. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Chen, C.; Xie, T.; Li, P. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Res. Int. 2020, 135, 109247. [Google Scholar] [CrossRef]
- Shao, X.; Xu, B.; Zhou, H.; Chen, C.; Li, P. Insight into the mechanism of decreasing N-nitrosodimethylamine by Lactobacillus pentosus R3 in a model system. Food Control 2020, 121, 107534. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V. Heat assisted HPP for the inactivation of bacteria, moulds and yeasts spores in foods: Log reductions and mathematical models. Trends Food Sci. Technol. 2019, 88, 143–156. [Google Scholar] [CrossRef]
- Zahid, A.; Choi, J.Y.; Seo, J.-K.; Parvin, R.; Ko, J.; Yang, H.-S. Effects of clove extract on oxidative stability and sensory attributes in cooked beef patties at refrigerated storage. Meat Sci. 2019, 161, 107972. [Google Scholar] [CrossRef]
- Fernandez, P.; Cofrades, S.; Solas, M.T.; Carballo, J.; Jimenez-Colmenero, F. High Pressure-Cooking of Chicken Meat Batters with Starch, Egg White, and Iota Carrageenan. J. Food Sci. 2008, 63, 267–271. [Google Scholar] [CrossRef]
- Chai, H.-E.; Sheen, S. Effect of high pressure processing, allyl isothiocyanate, and acetic acid stresses on Salmonella survivals, storage, and appearance color in raw ground chicken meat. Food Control 2020, 123, 107784. [Google Scholar] [CrossRef]
- Del Olmo, A.; Morales, P.; Ávila, M.; Calzada, J.; Nuñez, M. Effect of single-cycle and multiple-cycle high-pressure treatments on the colour and texture of chicken breast fillets. Innov. Food Sci. Emerg. Technol. 2010, 11, 441–444. [Google Scholar] [CrossRef]
- Sun, S.; Rasmussen, F.D.; Cavender, G.A.; Sullivan, G.A. Texture, color and sensory evaluation of sous-vide cooked beef steaks processed using high pressure processing as method of microbial control. LWT-Food Sci. Technol. 2018, 103, 169–177. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, S.; Yang, H.; Kamboh, A.A.; Ahmad, Z.; Tume, R.K.; Zhou, G. Improvement of color, texture and food safety of ready-to-eat high pressure-heat treated duck breast. Food Chem. 2018, 277, 646–654. [Google Scholar] [CrossRef]
- Udayasoorian, L.; Peter, M.; Sabina, K.; Indumathi, C.; Muthusamy, S. Comparative evaluation on shelf life extension of MAP packed Litopenaeus vannamei shrimp treated with natural extracts. LWT-Food Sci. Technol. 2017, 77, 217–224. [Google Scholar] [CrossRef]
- Krishnan, K.R.; Sivarajan, M.; Babuskin, S.; Archana, G.; Babu, P.A.S.; Sukumar, M. Kinetic modeling of spice extraction from S. aromaticum and C. cassia. J. Food Eng. 2013, 117, 326–332. [Google Scholar] [CrossRef]
- Torrecilhas, J.A.; Ornaghi, M.G.; Passetti, R.A.C.; Mottin, C.; Guerrero, A.; Ramos, T.R.; Vital, A.C.P.; Sañudo, C.; Malheiros, E.B.; Prado, I.N.D. Meat quality of young bulls finished in a feedlot and supplemented with clove or cinnamon essential oils. Meat Sci. 2020, 174, 108412. [Google Scholar] [CrossRef]
- Kameník, J.; Saláková, A.; Hulánková, R.; Borilova, G. The effect of high pressure on the microbiological quality and other characteristics of cooked sausages packed in a modified atmosphere or vacuum. Food Control 2015, 57, 232–237. [Google Scholar] [CrossRef]
- Cunha, L.; Monteiro, M.L.G.; Costa-Lima, B.R.; Guedes-Oliveira, J.M.; Alves, V.H.; Almeida, A.L.; Tonon, R.V.; Rosenthal, A.; Conte-Junior, C.A. Effect of microencapsulated extract of pitaya (Hylocereus costaricensis) peel on color, texture and oxidative stability of refrigerated ground pork patties submitted to high pressure processing. Innov. Food Sci. Emerg. Technol. 2018, 49, 136–145. [Google Scholar] [CrossRef]
- Orlien, V.; Hansen, E.; Skibsted, L.H. Lipid oxidation in high-pressure processed chicken breast muscle during chill storage: Critical working pressure in relation to oxidation mechanism. Eur. Food Res. Technol. 2000, 211, 99–104. [Google Scholar] [CrossRef]
- Bolumar, T.; LaPena, D.; Skibsted, L.H.; Orlien, V. Rosemary and oxygen scavenger in active packaging for prevention of high-pressure induced lipid oxidation in pork patties. Food Packag. Shelf Life 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Wood, J.; Enser, M.; Fisher, A.; Nute, G.; Sheard, P.; Richardson, I.; Hughes, S.; Whittington, F. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Soladoye, O.; Juárez, M.; Aalhus, J.; Shand, P.; Estevez, M. Protein Oxidation in Processed Meat: Mechanisms and Potential Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.; Sheen, S. High pressure processing of raw meat with essential oils-microbial survival, meat quality, and models: A review. Food Control 2021, 132, 108529. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Xiong, Y.L.; Alderton, A.L. Concentration effects of hydroxyl radical oxidizing systems on biochemical properties of porcine muscle myofibrillar protein. Food Chem. 2007, 101, 1239–1246. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Chen, H.; Diao, J.; Li, Y.; Chen, Q.; Kong, B. The effectiveness of clove extracts in the inhibition of hydroxyl radical oxidation-induced structural and rheological changes in porcine myofibrillar protein. Meat Sci. 2015, 111, 60–66. [Google Scholar] [CrossRef]
- Thanh, M.D.; Frentzel, H.; Fetsch, A.; Krause, G.; Appel, B.; Mader, A. Tenacity of Bacillus cereus and Staphylococcus aureus in dried spices and herbs. Food Control 2018, 83, 75–84. [Google Scholar] [CrossRef]
- Yin, L.; Chen, J.; Wang, K.; Geng, Y.; Lai, W.; Huang, X.; Chen, D.; Guo, H.; Fang, J.; Chen, Z.; et al. Study the antibacterial mechanism of cinnamaldehyde against drug-resistant Aeromonas hydrophila in vitro. Microb. Pathog. 2020, 145, 104208. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Y.; Hou, Q.; Huang, M.; Zhou, X. Dynamic changes of the bacterial communities in roast chicken stored under normal and modified atmosphere packaging. J. Food Sci. 2020, 85, 1231–1239. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Koidis, A. Suitability, efficiency and microbiological safety of novel physical technologies for the processing of ready-to-eat meals, meats and pumpable products. Int. J. Food Sci. Technol. 2015, 50, 1283–1302. [Google Scholar] [CrossRef]
- Alfaia, A.; Alfaia, C.; Patarata, L.; Fernandes, M.J.; Elias, M.; Ribeiro, M.; Fraqueza, M.J. Binomial effects of high isostatic pressure and time on the microbiological, sensory characteristics and lipid composition stability of vacuum packed dry fermented sausages “chouriço”. Innov. Food Sci. Emerg. Technol. 2015, 32, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Omer, M.K.; Alvseike, O.; Hoick, A.; Axelsson, L.; Prieto, M.; Skjerve, E.; Heir, E. Application of high pressure processing to reduce verotoxigenic E. coli in two types of dry-fermented sausage. Meat Sci. 2010, 86, 1005–1009. [Google Scholar] [CrossRef]
- Duranton, F.; Guillou, S.; Simonin, H.; Chéret, R.; de Lamballerie, M. Combined use of high pressure and salt or sodium nitrite to control the growth of endogenous microflora in raw pork meat. Innov. Food Sci. Emerg. Technol. 2012, 16, 373–380. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Argyri, A.A.; Papadopoulou, O.S.; Nisiotou, A.; Tassou, C.; Chorianopoulos, N. Effect of high pressure processing on the survival of Salmonella Enteritidis and shelf-life of chicken fillets. Food Microbiol. 2018, 70, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Karam, L.; Chehab, R.; Osaili, T.M.; Savvaidis, I.N. Antimicrobial effect of thymol and carvacrol added to a vinegar-based marinade for controlling spoilage of marinated beef (Shawarma) stored in air or vacuum packaging. Int. J. Food Microbiol. 2020, 332, 108769. [Google Scholar] [CrossRef]
- Han, Y.; Xu, X.; Jiang, Y.; Zhou, G.; Sun, X.; Xu, B. Inactivation of food spoilage bacteria by high pressure processing: Evaluation with conventional media and PCR–DGGE analysis. Food Res. Int. 2010, 43, 1719–1724. [Google Scholar] [CrossRef]
- Smith, C.J.; Olszewska, M.A.; Diez-Gonzalez, F. Selection and application of natural antimicrobials to control Clostridium perfringens in sous-vide chicken breasts inhibition of C. perfringens in sous-vide chicken. Int. J. Food Microbiol. 2021, 347, 109193. [Google Scholar] [CrossRef] [PubMed]
- McKee, L. Microbial contamination of spices and herbs: A review. LWT-Food Sci. Technol. 1995, 28, 1–11. [Google Scholar] [CrossRef]
- Lins, P. Production of Clostridium perfringensspores and their recovery from artificially spiked spices and herbs. J. Food Saf. 2018, 38, e12453. [Google Scholar] [CrossRef]





| Treatment | Storage Time (d) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | 20 | 24 | |
| L* value | |||||||
| Control | 97.82 ± 0.07 Aa | 97.69 ± 0.03 Aa | 97.69 ± 0.04 Aa | 97.62 ± 0.07 Aa | |||
| SE | 97.32 ± 0.05 BCa | 97.15 ± 0.08 BCab | 96.88 ± 0.09 Ca | 97 ± 0.14 Bab | 97.1 ± 0.14 Bab | ||
| HPP | 97.6 ± 0.11 ABa | 97.45 ± 0.07 ABa | 97.61 ± 0.04 Aa | 97.53 ± 0.17 Aa | 97.42 ± 0.17 Aa | 97.46 ± 0.1 Aa | 97.7 ± 0.05 Aa |
| SE-HPP | 97.07 ± 0.25 Ca | 96.94 ± 0.23 Ca | 97.22 ± 0.09 Bb | 96.78 ± 0.14 Ba | 96.76 ± 0.09 Ca | 96.92 ± 0.01 Ba | 96.9 ± 0.11 Ba |
| b* value | |||||||
| Control | 37.2 ± 0.84 Ba | 37.01 ± 0.4 Ba | 36.63 ± 1.0 Ba | 37.85 ± 0.52 Ba | |||
| SE | 37.87 ± 0.66 Bb | 39.88 ± 1.2 ABab | 41.04 ± 1.16 Aa | 41.58 ± 0.68 Aa | 42.71 ± 0.61 Aa | ||
| HPP | 38.4 ± 0.27 Ba | 38.09 ± 1.18 ABa | 36.09 ± 1.01 Bab | 36.82 ± 1.77 Ba | 37.8 ± 1.44 Ba | 37.23 ± 0.82 Ba | 32.91 ± 0.6 Ba |
| SE-HPP | 41.6 ± 0.66 Aa | 43.4 ± 3.21 Aa | 43.18 ± 0.99 Aa | 44.43 ± 0.88 Aa | 45.8 ± 1.19 Aa | 42.86 ± 0.11 Aa | 43.64 ± 0.83 Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Q.; Xu, M.; Xu, B.; Chen, C.; Deng, J.; Li, P. Combined Effect of High-Pressure Processing with Spice Extracts on Quality of Low-Salt Sausage during Refrigerated Storage. Foods 2021, 10, 2610. https://doi.org/10.3390/foods10112610
Xiao Q, Xu M, Xu B, Chen C, Deng J, Li P. Combined Effect of High-Pressure Processing with Spice Extracts on Quality of Low-Salt Sausage during Refrigerated Storage. Foods. 2021; 10(11):2610. https://doi.org/10.3390/foods10112610
Chicago/Turabian StyleXiao, Qing, Mei Xu, Baocai Xu, Conggui Chen, Jieying Deng, and Peijun Li. 2021. "Combined Effect of High-Pressure Processing with Spice Extracts on Quality of Low-Salt Sausage during Refrigerated Storage" Foods 10, no. 11: 2610. https://doi.org/10.3390/foods10112610
APA StyleXiao, Q., Xu, M., Xu, B., Chen, C., Deng, J., & Li, P. (2021). Combined Effect of High-Pressure Processing with Spice Extracts on Quality of Low-Salt Sausage during Refrigerated Storage. Foods, 10(11), 2610. https://doi.org/10.3390/foods10112610