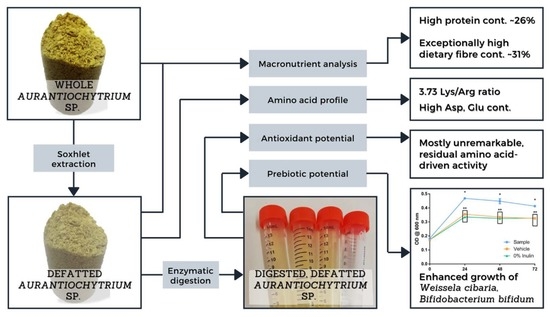
Evaluating the Potential of the Defatted By-Product of Aurantiochytrium sp. Industrial Cultivation as a Functional Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recovery of Spent Aurantiochytrium sp. Biomass
2.2. Chemical Analysis of the Spent Aurantiochytrium sp. Biomass
2.3. Enzymatic Digestion of the Spent Aurantiochytrium sp. Biomass
2.4. In Vitro Prebiotic Potential Assay
2.5. Antioxidant and Lipid Oxidation Protective Assays
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis
3.2. Amino Acid Profile
3.3. Antioxidant and Lipid Protective Activities
3.4. Prebiotic Potential
3.4.1. Method Validation
3.4.2. Growth Effect on Probiotic Cultures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, X.M.; Xu, Y.S.; Huang, H. Thraustochytrid Cell Factories for Producing Lipid Compounds. Trends Biotechnol. 2020, 65, 102–113. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Mining of squalene as a value-added byproduct from DHA producing marine thraustochytrid cultivated on food waste hydrolysate. Sci. Total Environ. 2020, 736, 139691. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Thilakaratne, R.; Brown, R.C.; Wright, M.M. Techno-economic analysis of transportation fuels from defatted microalgae via hydrothermal liquefaction and hydroprocessing. Biomass Bioenergy 2015, 72, 45–54. [Google Scholar] [CrossRef]
- Aida, T.M.; Maruta, R.; Tanabe, Y.; Oshima, M.; Nonaka, T.; Kujiraoka, H.; Kumagai, Y.; Ota, M.; Suzuki, I.; Watanabe, M.M.; et al. Nutrient recycle from defatted microalgae (Aurantiochytrium) with hydrothermal treatment for microalgae cultivation. Bioresour. Technol. 2017, 228, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.R.; Aloui, H.; Khomlaem, C.; Negi, A.; Yun, J.H.; Kim, H.S.; Kim, B.S. Biodegradable films based on chitosan and defatted Chlorella biomass: Functional and physical characterization. Food Chem. 2021, 337, 127777. [Google Scholar] [CrossRef]
- Medina, C.; Rubilar, M.; Shene, C.; Torres, S.; Verdugo, M. Protein fractions with techno-functional and antioxidant properties from Nannochloropsis gaditana microalgal biomass. J. Biobased Mater. Bioenergy 2015, 9, 417–425. [Google Scholar] [CrossRef]
- Gatrell, S.; Lum, K.; Kim, J.; Lei, X.G. Nonruminant nutrition symposium: Potential of defatted microalgae from the biofuel industry as an ingredient to replace corn and soybean meal in swine and poultry diets. J. Anim. Sci. 2014, 92, 1306–1314. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursa’cbursa´c, D.; Kovačevi´c, K.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Annual Review of Food Science and Technology Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Ziemer, C.J.; Gibson, G.R. An overview of probiotics, prebiotics and synbiotics in the functional food concept: Perspectives and future strategies. Proc. Int. Dairy J. 1998, 8, 473–479. [Google Scholar] [CrossRef]
- Kim, B.; Hong, V.M.; Yang, J.; Hyun, H.; Im, J.J.; Hwang, J.; Yoon, S.; Kim, J.E. A review of fermented foods with beneficial effects on brain and cognitive function. Prev. Nutr. Food Sci. 2016, 21, 297–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, A.K.; Pandey, A.; Sahoo, D. Biotechnological potential of yeasts in functional food industry. Trends Food Sci. Technol. 2019, 83, 129–137. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Successful approaches for a red seaweed biorefinery. Mar. Drugs 2019, 17, 620. [Google Scholar] [CrossRef] [Green Version]
- Imbimbo, P.; D’Elia, L.; Liberti, D.; Olivieri, G.; Monti, D.M. Towards green extraction methods from microalgae learning from the classics. Appl. Microbiol. Biotechnol. 2020, 104, 9067–9077. [Google Scholar] [CrossRef] [PubMed]
- Adebola, O.O.; Corcoran, O.; Morgan, W.A. Synbiotics: The impact of potential prebiotics inulin, lactulose and lactobionic acid on the survival and growth of lactobacilli probiotics. J. Funct. Foods 2014, 10, 75–84. [Google Scholar] [CrossRef]
- Pham, V.T.; Mohajeri, M.H. The application of in vitro human intestinal models on the screening and development of pre-And probiotics. Benef. Microbes 2018, 9, 725–742. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.L.; Hsieh, S.L.; Chen, C.W.; Dong, C. Di Emerging prospects of macro- and microalgae as prebiotic. Microb. Cell Fact. 2021, 20, 1–16. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Kim, J.M.; Jin, Z.; Zhou, J. Prebiotic effectiveness of inulin extracted from edible burdock. Anaerobe 2008, 14, 29–34. [Google Scholar] [CrossRef]
- Zheng, L.X.; Chen, X.Q.; Cheong, K.L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef] [PubMed]
- de la Peña, M.R.; Teruel, M.B.; Oclarit, J.M.; Amar, M.J.A.; Ledesma, E.G.T. Use of thraustochytrid Schizochytrium sp. as source of lipid and fatty acid in a formulated diet for abalone Haliotis asinina (Linnaeus) juveniles. Aquac. Int. 2016, 24, 1103–1118. [Google Scholar] [CrossRef]
- Burja, A.M.; Armenta, R.E.; Radianingtyas, H.; Barrow, C.J. Evaluation of fatty acid extraction methods for Thraustochytrium sp. ONC-T18. J. Agric. Food Chem. 2007, 55, 4795–4801. [Google Scholar] [CrossRef] [PubMed]
- Tada, S.; Innami, S. A Simplified Modification of the AOAC Official Method for Determination of Total Dietary Fiber Using Newly Developed Enzymes. J. AOAC Int. 2007, 90, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef]
- Vallabha, V.S.; Tapal, A.; Sukhdeo, S.V.; Govindaraju, K.; Tiku, P.K. Effect of arginine:lysine ratio in free amino acid and protein form on l-NAME induced hypertension in hypercholesterolemic Wistar rats. RSC Adv. 2016, 6, 73388–73398. [Google Scholar] [CrossRef]
- Bidlingmeyer, B.A.; Cohen, S.A.; Tarvin, T.L. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. B Biomed. Sci. Appl. 1984, 336, 93–104. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Lin, R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT-Food Sci. Technol. 2009, 42, 137–143. [Google Scholar] [CrossRef]
- Wichienchot, S.; Jatupornpipat, M.; Rastall, R.A. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 2010, 120, 850–857. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Huang, G.; Zhang, W.; Ni, L. In vitro and in vivo evaluation of the prebiotic effect of raw and roasted almonds (Prunus amygdalus). J. Sci. Food Agric. 2016, 96, 1836–1843. [Google Scholar] [CrossRef] [Green Version]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Hsieh, C.L. Antioxidant Activity of Extracts from Du-zhong (Eucommia ulmoides) toward Various Lipid Peroxidation Models in Vitro. J. Agric. Food Chem. 1998, 46, 3952–3957. [Google Scholar] [CrossRef]
- Félix, R.; Carmona, A.M.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Industry-friendly hydroethanolic extraction protocols for grateloupia turuturu UV-shielding and antioxidant compounds. Appl. Sci. 2020, 10, 5304. [Google Scholar] [CrossRef]
- Félix, R.; Valentão, P.; Andrade, P.B.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Evaluating the in vitro potential of natural extracts to protect lipids from oxidative damage. Antioxidants 2020, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Trovão, M.; Pereira, H.; Costa, M.; Machado, A.; Barros, A.; Soares, M.; Carvalho, B.; Silva, J.T.; Varela, J.; Silva, J.T. Lab-Scale Optimization of Aurantiochytrium sp. Culture Medium for Improved Growth and DHA Production. Appl. Sci. 2020, 10, 2500. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.G.; Kim, K.; Kim, J.; Han, J.I.; Yang, J.W. Use of organic waste from the brewery industry for high-density cultivation of the docosahexaenoic acid-rich microalga, Aurantiochytrium sp. KRS101. Bioresour. Technol. 2013, 129, 351–359. [Google Scholar] [CrossRef]
- Jakobsen, A.N.; Aasen, I.M.; Josefsen, K.D.; Strøm, A.R. Accumulation of docosahexaenoic acid-rich lipid in thraustochytrid Aurantiochytrium sp. strain T66: Effects of N and P starvation and O2 limitation. Appl. Microbiol. Biotechnol. 2008, 80, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Sami, R.; Lianzhou, J.; Yang, L.; Ma, Y.; Jing, J. Evaluation of fatty acid and amino acid compositions in okra (abelmoschus esculentus) grown in different geographical locations. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.A.; Morlacchini, M.; Keegan, J.D.; Fusconi, G. Increasing the Omega-3 Content of Hen’s Eggs Through Dietary Supplementation with Aurantiochytrium limacinum Microalgae: Effect of Inclusion Rate on the Temporal Pattern of Docosahexaenoic Acid Enrichment, Efficiency of Transfer, and Egg Characteristics. J. Appl. Poult. Res. 2019, 28, 329–338. [Google Scholar] [CrossRef]
- Tran, T.L.N.; Miranda, A.F.; Mouradov, A.; Adhikari, B. Physicochemical characteristics of protein isolated from thraustochytrid oilcake. Foods 2020, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Vega-López, S.; Matthan, N.R.; Ausman, L.M.; Harding, S.V.; Rideout, T.C.; Ai, M.; Otokozawa, S.; Freed, A.; Kuvin, J.T.; Jones, P.J.; et al. Altering dietary lysine:arginine ratio has little effect on cardiovascular risk factors and vascular reactivity in moderately hypercholesterolemic adults. Atherosclerosis 2010, 210, 555–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Chen, J.; Xu, T.; Qiu, W.; Zhang, Y.; Zhang, L.; Xu, F.; Liu, H. Rice protein extracted by different methods affects cholesterol metabolism in rats due to its lower digestibility. Int. J. Mol. Sci. 2011, 12, 7594–7608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temussi, P.A. The good taste of peptides. J. Pept. Sci. 2012, 18, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, X.; Wang, L.; Cui, Q. Comprehensive analysis of metabolic alterations in Schizochytrium sp. strains with different DHA content. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1160, 122193. [Google Scholar] [CrossRef] [PubMed]
- De Melo, M.M.R.; Sapatinha, M.; Pinheiro, J.; Lemos, M.F.L.; Bandarra, N.M.; Batista, I.; Paulo, M.C.; Coutinho, J.; Saraiva, J.A.; Portugal, I.; et al. Supercritical CO2 extraction of Aurantiochytrium sp. biomass for the enhanced recovery of omega-3 fatty acids and phenolic compounds. J. CO2 Util. 2020, 38, 24–31. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.H. Significance of Dietary Antioxidants for Health. Int. J. Mol. Sci. 2011, 13, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Antioxidant active packaging for chicken meat processed by high pressure treatment. Food Chem. 2011, 129, 1406–1412. [Google Scholar] [CrossRef]
- Ricciardi, A.; Parente, E.; Zotta, T. Modelling the growth of Weissella cibaria as a function of fermentation conditions. J. Appl. Microbiol. 2009, 107, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.W.; Liu, Z.S.; Kuo, T.C.; Hsieh, M.C.; Li, Z.W. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. BioMetals 2017, 30, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Goderska, K. The antioxidant and prebiotic properties of lactobionic acid. Appl. Microbiol. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, R.; Sakanaka, M.; Yoshimi, K.; Sugimoto, N.; Eguchi, S.; Yamauchi, Y.; Nara, M.; Maeda, S.; Ami, Y.; Gotoh, A.; et al. Next-generation prebiotic promotes selective growth of bifidobacteria, suppressing Clostridioides difficile. Gut Microbes 2021, 13, 1973835. [Google Scholar] [CrossRef]



| Sample ID | Lipid Content (g/100 g) | Protein Content (g/100 g) | Ash Content (g/100 g) | Fibre Content (g/100 g) |
|---|---|---|---|---|
| WA | 42.7 ± 0.8 | 15.3 ± 0.7 | 10.7 ± 0.1 | 17.8 ± 1.5 |
| DA | 2.4 ± 0.8 | 26.7 ± 1.8 | 16.9 ± 0.9 | 31.0 ± 1.1 |
| Amino Acid | Aurantiochytrium sp. Spent Biomass (g/100 g) | % of Total AA | Aurantiochytrium sp. from Literature (g/100 g) | References (Aurantiochytrium) | Thraustochytrids from Literature (g/100 g) | References (Thraustochytrids) | ||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | |||||
| Essential | ||||||||
| Alanine | 2.2 | 4.0 | 0.8 | 3.9 | [4,40] | 0.53 | 7.5 | [38,40,44] |
| Arginine | 1.1 | 2.0 | 5.5 | 12.3 | 0.67 | 12.3 | ||
| Aspartic acid | 7.0 | 12.7 | 5.6 | 7.1 | 2.91 | 14.7 | ||
| Glutamic acid | 18 | 32.5 | 11.2 | 11.4 | 1.74 | 42.0 | ||
| Glycine | 2.2 | 4.0 | 1.3 | 1.5 | 0.38 | 7.0 | ||
| Histidine | 1.1 | 2.0 | 8.6 | 10.3 | 0.23 | 1.1 | ||
| Serine | 4.2 | 7.6 | 2.6 | 3.2 | 0.46 | 10.8 | ||
| Threonine | 1.8 | 3.3 | 0.3 | 0.8 | 0.38 | 1.5 | ||
| Non-essential | ||||||||
| Cysteine | 0.79 | 1.4 | 0.3 | 0.4 | [4,40] | 0.14 | 1.4 | [38,40,44] |
| Isoleucine | 1.6 | 2.9 | 1.8 | 2.4 | 0.22 | 2.3 | ||
| Leucine | 3.3 | 6.0 | 3.7 | 4.8 | 0.56 | 6.8 | ||
| Lysine | 4.1 | 7.4 | 3.9 | 5.0 | 0.5 | 7.2 | ||
| Methionine | 2.4 | 4.3 | 1.1 | 1.1 | 0.05 | 1.8 | ||
| Phenylalanine | 1.4 | 2.5 | 2.1 | 2.7 | 0.36 | 3.7 | ||
| Proline | 3.2 | 5.8 | 2.6 | 2.7 | 1.38 | 3.6 | ||
| Valine | 0.93 | 1.7 | 2.7 | 3.4 | 0.34 | 4.0 | ||
| Sample ID | DPPH (mM DPPH/mL) | FRAP (Fe(II) eq (mM)/mL) | LPIP (% of Ctrl) |
|---|---|---|---|
| Digested DA | 0.025 ± 0.022 | 152.5 ± 6.2 | 162.1 ± 6.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reboleira, J.; Félix, R.; Félix, C.; de Melo, M.M.R.; Silva, C.M.; Saraiva, J.A.; Bandarra, N.M.; Teixeira, B.; Mendes, R.; Paulo, M.C.; et al. Evaluating the Potential of the Defatted By-Product of Aurantiochytrium sp. Industrial Cultivation as a Functional Food. Foods 2021, 10, 3058. https://doi.org/10.3390/foods10123058
Reboleira J, Félix R, Félix C, de Melo MMR, Silva CM, Saraiva JA, Bandarra NM, Teixeira B, Mendes R, Paulo MC, et al. Evaluating the Potential of the Defatted By-Product of Aurantiochytrium sp. Industrial Cultivation as a Functional Food. Foods. 2021; 10(12):3058. https://doi.org/10.3390/foods10123058
Chicago/Turabian StyleReboleira, João, Rafael Félix, Carina Félix, Marcelo M. R. de Melo, Carlos M. Silva, Jorge A. Saraiva, Narcisa M. Bandarra, Bárbara Teixeira, Rogério Mendes, Maria C. Paulo, and et al. 2021. "Evaluating the Potential of the Defatted By-Product of Aurantiochytrium sp. Industrial Cultivation as a Functional Food" Foods 10, no. 12: 3058. https://doi.org/10.3390/foods10123058
APA StyleReboleira, J., Félix, R., Félix, C., de Melo, M. M. R., Silva, C. M., Saraiva, J. A., Bandarra, N. M., Teixeira, B., Mendes, R., Paulo, M. C., Coutinho, J., & Lemos, M. F. L. (2021). Evaluating the Potential of the Defatted By-Product of Aurantiochytrium sp. Industrial Cultivation as a Functional Food. Foods, 10(12), 3058. https://doi.org/10.3390/foods10123058