Development of a Methodology for Estimating the Ergosterol in Meat Product-Borne Toxigenic Moulds to Evaluate Antifungal Agents
Abstract
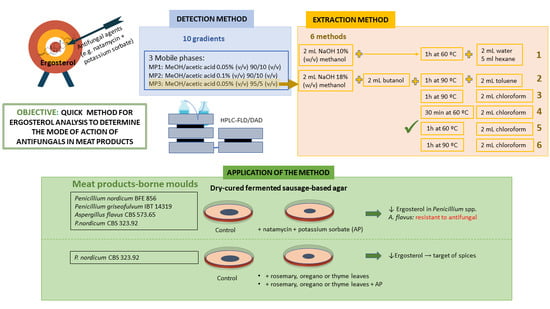
:1. Introduction
2. Materials and Methods
2.1. Standard and Reagents
2.2. Preparation of Standard Solutions
2.3. HPLC Method
2.4. Extraction of Ergosterol
2.5. Validation Assays
2.6. Mould Assessment
2.7. Statistical Analysis
3. Results and Discussion
3.1. Improvement of the Chromatographic Conditions
3.2. Extraction Method
3.3. Validation Parameters
3.4. Ergosterol Content of Toxigenic Moulds in the Presence of Antifungal Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zadravec, M.; Vahčić, N.; Brnić, D.; Markov, K.; Frece, J.; Beck, R.; Lešić, T.; Pleadin, J. A study of surface moulds and mycotoxins in Croatian traditional dry-cured meat products. Int. J. Food Microbiol. 2020, 317, 108459. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montero, L.; Córdoba, J.J.; Peromingo, B.; Álvarez, M.; Núñez, F. Effects of environmental conditions and substrate on growth and ochratoxin A production by Penicillium verrucosum and Penicillium nordicum: Relative risk assessment of OTA in dry-cured meat products. Food Res. Int. 2019, 121, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, N.; Kocsubé, S.; Varga, J. Aflatoxins: Climate change and biodegradation. Curr. Opin. Food Sci. 2015, 5, 60–66. [Google Scholar] [CrossRef]
- Peromingo, B.; Sulyok, M.; Lemmens, M.; Rodríguez, A.; Rodríguez, M. Diffusion of mycotoxins and secondary metabolites in dry-cured meat products. Food Control. 2019, 101, 144–150. [Google Scholar] [CrossRef]
- Payne, G.A. Mycotoxins and Product Safety. In Peanuts; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 347–361. [Google Scholar]
- Pattono, D.; Grosso, A.; Stocco, P.; Pazzi, M.; Zeppa, G. Survey of the presence of patulin and ochratoxin A in traditional semi-hard cheeses. Food Control. 2013, 33, 54–57. [Google Scholar] [CrossRef]
- Peromingo, B.; Rodríguez, M.; Núñez, F.; Silva, A.; Rodríguez, A. Sensitive determination of cyclopiazonic acid in dry-cured ham using a QuEChERS method and UHPLC–MS/MS. Food Chem. 2018, 263, 275–282. [Google Scholar] [CrossRef]
- Chaves-López, C.; Martin-Sánchez, A.M.; Fuentes-Zaragoza, E.; Viuda-Martos, M.; Fernández-López, J.; Sendra, E.; Sayas, E.; Alvarez, J.A.P. Role of Oregano (Origanum vulgare) Essential Oil as a Surface Fungus Inhibitor on Fermented Sausages: Evaluation of Its Effect on Microbial and Physicochemical Characteristics. J. Food Prot. 2012, 75, 104–111. [Google Scholar] [CrossRef]
- Álvarez, M.; Rodríguez, A.; Peromingo, B.; Núñez, F.; Rodríguez, M. Enterococcus faecium: A promising protective culture to control growth of ochratoxigenic moulds and mycotoxin production in dry-fermented sausages. Mycotoxin Res. 2019, 36, 137–145. [Google Scholar] [CrossRef]
- Andrade, M.J.; Thorsen, L.; Rodríguez, A.; Córdoba, J.J.; Jespersen, L. Inhibition of ochratoxigenic moulds by Debaryomyces hansenii strains for biopreservation of dry-cured meat products. Int. J. Food Microbiol. 2014, 170, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Peromingo, B.; Rodríguez, A.; Rodríguez, M. Biocontrol of Penicillium griseofulvum to reduce cyclopiazonic acid contamination in dry-fermented sausages. Int. J. Food Microbiol. 2019, 293, 1–6. [Google Scholar] [CrossRef]
- Álvarez, M.; Núñez, F.; Delgado, J.; Andrade, M.J.; Rodríguez, M.; Rodríguez, A. Competitiveness of three biocontrol candidates against ochratoxigenic Penicillium nordicum under dry-cured meat environmental and nutritional conditions. Fungal Biol. 2021, 125, 134–142. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off. J. Eur. Union. 2011. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.; Dimić, G.; Đerić, N.; Mojović, L.; Tomović, V.; Šojić, B.; Đukić-Vuković, A.; Pejin, J. Growth control of molds isolated from smoked fermented sausages using basil and caraway essential oils, in vitro and in vivo. LWT 2020, 123, 109095. [Google Scholar] [CrossRef]
- Álvarez, M.; Rodríguez, A.; Núñez, F.; Silva, A.; Andrade, M.J. In vitro antifungal effects of spices on ochratoxin A production and related gene expression in Penicillium nordicum on a dry-cured fermented sausage medium. Food Control. 2020, 114, 107222. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B. UV-based evaluation of ergosterol for monitoring the fungal exposure in Italian buffalo farms. FEMS Microbiol. Lett. 2017, 364, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Welscher, Y.M.T.; Napel, H.H.T.; Balagué, M.M.; Souza, C.M.; Riezman, H.; De Kruijff, B.; Breukink, E. Natamycin Blocks Fungal Growth by Binding Specifically to Ergosterol without Permeabilizing the Membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef] [Green Version]
- Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K.; Waśkiewicz, A. The Inhibitory Potential of Selected Essential Oils on Fusarium spp. Growth and Mycotoxins Biosynthesis in Maize Seeds. Pathogens 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandão, R.M.; Ferreira, V.R.F.; Batista, L.R.; Alves, E.; Lira, N.D.A.; Bellete, B.S.; Scolforo, J.R.S.; Cardoso, M.D.G. Antifungal and antimycotoxigenic effect of the essential oil of Eremanthus erythropappus on three different Aspergillus species. Flavour Fragr. J. 2020, 35, 524–533. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The Fungicidal Activity of Thymol against Fusarium graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jiang, N.; Wang, D.; Wang, M. Effects of Essential Oil Citral on the Growth, Mycotoxin Biosynthesis and Transcriptomic Profile of Alternaria alternata. Toxins 2019, 11, 553. [Google Scholar] [CrossRef] [Green Version]
- Kadakal, Ç.; Tepe, T.K. Is ergosterol a new microbiological quality parameter in foods or not? Food Rev. Int. 2019, 35, 155–165. [Google Scholar] [CrossRef]
- Pastinen, O.; Nyyssölä, A.; Pihlajaniemi, V.; Sipponen, M.H. Fractionation process for the protective isolation of ergosterol and trehalose from microbial biomass. Process. Biochem. 2017, 58, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Neuhof, T.; Koch, M.; Rasenko, T.; Nehls, I. Distribution of Trichothecenes, Zearalenone, and Ergosterol in a Fractionated Wheat Harvest Lot. J. Agric. Food Chem. 2008, 56, 7566–7571. [Google Scholar] [CrossRef]
- Mille-Lindblom, C.; Von Wachenfeldt, E.; Tranvik, L.J. Ergosterol as a measure of living fungal biomass: Persistence in environmental samples after fungal death. J. Microbiol. Methods 2004, 59, 253–262. [Google Scholar] [CrossRef]
- Dong, Y.; Steffenson, B.J.; Mirocha, C.J. Analysis of Ergosterol in Single Kernel and Ground Grain by Gas Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 4121–4125. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a Novel Methodology for the Analysis of Ergosterol in Mushrooms. Food Anal. Methods 2014, 7, 217–223. [Google Scholar] [CrossRef]
- Steel, C.; Schwarz, L.; Qiu, Y.; Schueuermann, C.; Blackman, J.; Clark, A.; Schmidtke, L. Thresholds for Botrytis bunch rot contamination of Chardonnay grapes based on the measurement of the fungal sterol, ergosterol. Aust. J. Grape Wine Res. 2019, 26, 79–89. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Morales-Soto, A.; Segura-Carretero, A.; Valverde, J. Potential of RP-UHPLC-DAD-MS for the qualitative and quantitative analysis of sofosbuvir in film coated tablets and profiling degradants. J. Pharm. Anal. 2017, 7, 208–213. [Google Scholar] [CrossRef]
- Lohr, D.; Woeck, C.; Meinken, E. Use of ergosterol as an indicator for colonization of peat-based growing media by saprophytic fungi. Eur. J. Hortic. Sci. 2017, 82, 3–11. [Google Scholar] [CrossRef]
- Parkinson, D.-R.; Warren, J.; Pawliszyn, J. Analysis of ergosterol for the detection of mold in soils by automated on-fiber derivatization headspace extraction–SPME-GC/MS. Anal. Chim. Acta 2010, 661, 181–187. [Google Scholar] [CrossRef]
- Porep, J.U.; Mrugala, S.; Pour Nikfardjam, M.S.; Carle, R. Online Determination of Ergosterol in Naturally Contaminated Grape Mashes Under Industrial Conditions at Wineries. Food Bioprocess Technol. 2015, 8, 1455–1464. [Google Scholar] [CrossRef]
- Food and Drug Administration. Bioanalytical Method Validation: Guidance for Industry. In Rockville, MD: CDER; FDA: Beltsville, MD, USA, 2018; Volume FDA-2013-D-1020. [Google Scholar]
- ICH Guideline M10 on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-ich-guideline-m10-bioanalytical-method-validation-step-2b_en.pdf (accessed on 4 November 2020).
- Hayash, Y.; Matsuda, R. Deductive Prediction of Measurement Precision from Signal and Noise in Photomultiplier and Photodiode Detector of Liquid Chromatography. Anal. Sci. 1994, 10, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Introduction to Modern Liquid Chromatography; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Sergeeva, Y.E.; Galanina, L.A.; Kochkina, G.A.; Feofilova, E.P. The effect of the preservative sorbic acid on the lipid composition of the ascomycete fungus Penicillium roqueforti Thom. Microbiology 2009, 78, 630–635. [Google Scholar] [CrossRef]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 2008, 39, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streekstra, H.; Verkennis, A.E.; Jacobs, R.; Dekker, A.; Stark, J.; Dijksterhuis, J. Fungal strains and the development of tolerance against natamycin. Int. J. Food Microbiol. 2016, 238, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, M.; Aldred, D.; Magan, N. Environmental factors and weak organic acid interactions have differential effects on control of growth and ochratoxin A production by Penicillium verrucosum isolates in bread. Int. J. Food Microbiol. 2005, 98, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Bills, C.E. Vitamin D Group. In The Vitamins Chemistry, Physiology and Pathology; Sebrel, W.H., Harris, R.S., Eds.; Academic Press: New York, NY, USA, 1954; pp. 131–266. [Google Scholar]
- Newell, S.Y.; Arsuffi, T.L.; Fallon, R.D. Fundamental procedures for determining ergosterol content of decaying plant ma-terial by liquid chromatography. Appl. Environ. Microbiol. 1988, 54, 1876–1879. [Google Scholar] [CrossRef] [Green Version]
- Kaale, E.; Shewiyo, D.H.; Jenkins, D. Statistics in validation of quantitative chromatographic methods. In Chemometrics in Chromatography; Komsta, Ł., Vander Heyden, Y., Sherma, J., Eds.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2018. [Google Scholar]
- ICH Validation of Analytical Procedures: Text and Methodology. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 4 November 2020).
- Raposo, F. Evaluation of analytical calibration based on least-squares linear regression for instrumental techniques: A tutorial review. TrAC Trends Anal. Chem. 2016, 77, 167–185. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Hocking, A.D.; Fleet, G.H. Comparison of hyphal length, ergosterol, mycelium dry weight, and colony diameter for quantifying growth of fungi from foods. Adv. Exp. Med. Biol. 2006, 571, 49–67. [Google Scholar] [CrossRef]
- Saxena, J.; Munimbazi, C.; Bullerman, L.B. Relationship of mould count, ergosterol and ochratoxin A production. Int. J. Food Microbiol. 2001, 71, 29–34. [Google Scholar] [CrossRef]
- Van Leeuwen, M.; Smant, W.; De Boer, W.; Dijksterhuis, J. Filipin is a reliable in situ marker of ergosterol in the plasma membrane of germinating conidia (spores) of Penicillium discolor and stains intensively at the site of germ tube formation. J. Microbiol. Methods 2008, 74, 64–73. [Google Scholar] [CrossRef]
- Geißel, B.; Loiko, V.; Klugherz, I.; Zhu, Z.; Wagener, N.; Kurzai, O.; Hondel, C.A.M.J.J.V.D.; Wagener, J. Azole-induced cell wall carbohydrate patches kill Aspergillus fumigatus. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bomfim, N.D.S.; Kohiyama, C.Y.; Nakasugi, L.P.; Nerilo, S.B.; Mossini, S.A.G.; Romoli, J.C.Z.; Mikcha, J.M.G.; Filho, B.A.D.A.; Machinski, M., Jr. Antifungal and antiaflatoxigenic activity of rosemary essential oil (Rosmarinus officinalis L.) against Aspergillus flavus. Food Addit. Contam. Part A 2019, 37, 153–161. [Google Scholar] [CrossRef]
- Bomfim, N.D.S.; Nakassugi, L.P.; Oliveira, J.F.P.; Kohiyama, C.Y.; Mossini, S.A.G.; Grespan, R.; Nerilo, S.B.; Mallmann, C.A.; Filho, B.A.A.; Machinski, M. Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem. 2015, 166, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Chun, H.S. p-Cymene and its derivatives exhibit antiaflatoxigenic activities against Aspergillus flavus through multiple modes of action. Appl. Biol. Chem. 2018, 61, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shao, X.; Xu, J.; Wei, Y.; Xu, F.; Wang, H. Effects and possible mechanism of tea tree oil against Botrytis cinerea and Penicillium expansum in vitro and in vivo test. Can. J. Microbiol. 2017, 63, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Guha, P. Use of predictive model to describe sporicidal and cell viability efficacy of betel leaf (Piper betle L.) essential oil on Aspergillus flavus and Penicillium expansum and its antifungal activity in raw apple juice. LWT 2017, 80, 510–516. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.; Yang, H.; Yuan, Y.; Yue, T. Antifungal mechanism of cinnamaldehyde and citral combination against Penicillium expansum based on FT-IR fingerprint, plasma membrane, oxidative stress and volatile profile. RSC Adv. 2018, 8, 5806–5815. [Google Scholar] [CrossRef] [Green Version]
- Xin, Z.; Ouyang, Q.; Wan, C.; Che, J.; Li, L.; Chen, J.; Tao, N. Isolation of antofine from Cynanchum atratum BUNGE (Asclepiadaceae) and its antifungal activity against Penicillium digitatum. Postharvest Biol. Technol. 2019, 157, 110961. [Google Scholar] [CrossRef]
- Hua, H.; Xing, F.; Selvaraj, J.N.; Wang, Y.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Inhibitory Effect of Essential Oils on Aspergillus ochraceus Growth and Ochratoxin A Production. PLoS ONE 2014, 9, e108285. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Methods | Main Steps Involved in the Procedure | Recovery (%) | Standard Deviation (%) 2 | Retention Time (min) | References |
|---|---|---|---|---|---|
| Method 1 | 2 mL NaOH 10% (w/v) in methanol + vortex 30 s + 1 h at 60 °C + 2 mL distilled water + 5 mL hexane + evaporation of the hexane extract + resuspension in 1 mL MP 1 | 9.11 * | ±6.97 | 16.7 | [24] |
| Method 2 | 2 mL NaOH 18% (w/v) in distilled water + 2 mL 1-butanol + vortex 30 s + 1 h at 90 °C + 2 mL toluene + centrifugation (5000 rpm for 5 min) + evaporation of the organic phase + resuspension in 1 mL MP | 47.45 * | ±73.69 | 16.4 | [23] |
| Method 3 | 2 mL NaOH 18% (w/v) in distilled water + 2 mL 1-butanol + vortex 30 s + 1 h at 90 °C + 2 mL chloroform + centrifugation (5000 rpm for 5 min) + evaporation of the organic phase + resuspension in 1 mL MP | 103.10 | ±26.97 | 15.8 | This study |
| Method 4 | 2 mL NaOH 18% (w/v) in distilled water + 2 mL 1-butanol + vortex 30 s + 30 min at 90 °C + 2 mL chloroform + centrifugation (5000 rpm for 5 min) + evaporation of the organic phase + resuspension in 1 mL MP | 82.12 | ±80.37 | 15.9 | This study |
| Method 5 | 2 mL NaOH 18% (w/v) in distilled water + 2 mL 1-butanol + vortex 30 s + 1 h at 60 °C + 2 mL chloroform + centrifugation (5000 rpm for 5 min) + evaporation of the organic phase + resuspension in 1 mL MP | 99.51 | ±9.92 | 15.8 | This study |
| Method 6 | 2 mL NaOH 18% (w/v) in distilled water + 2 mL 1-butanol + vortex 30 s + 1 h at 90 °C + 3 mL chloroform + centrifugation (5000 rpm for 5 min) + evaporation of the organic phase + resuspension in 1 mL MP | 81.09 * | ±21.63 | 15.9 | This study |
| Mobile Phases 2 | Retention Time (min) | Coefficient of Variations (%) 3 | Width of the Peaks (min) | Asymmetry |
|---|---|---|---|---|
| MP1 | 17.43 | 12.75 | 0.24 | 0.90 |
| MP2 | 17.33 | 8.79 | 0.23 | 0.91 |
| MP3 | 15.44 | 7.59 | 0.18 | 0.97 |
| AP (%, v/v) | Concentration of Ergosterol | ||
|---|---|---|---|
| 0 h | 8 h | 24 h | |
| 0 | 8.74 ± 1.28 1 | 8.44 ± 1.60 | 9.11 ± 0.72 |
| 10 | 9.28 ± 0.71 | 8.85 ± 0.56 | 9.03 ± 0.69 |
| 50 | 0.50 ± 0.43 * | n.d * | n.d * |
| 90 | n.d 2* | n.d * | n.d * |
| Mould Strain | ||||
|---|---|---|---|---|
| Penicillium nordicum CBS 323.92 | P. nordicum BFE 856 | Penicillium griseofulvum IBT 14319 | Aspergillus flavus CBS 573.65 | |
| Control | 731.55 ± 183.54 1 | 365.57 ± 45.96 | 1121.61 ± 486.34 | 248.09 ± 174.49 |
| Mould + AP | 513.59 ± 92.84 * | 187.15 ± 92.14 * | 185.89 ± 158.75 * | 297.22 ± 207.47 |
| Treatment 1 | Ergosterol Content |
|---|---|
| FS | 731.55 ± 183.54 2 |
| FS-R | 184.34 ± 44.50 * |
| FS-O | 177.40 ± 17.50 * |
| FS-T | 117.70 ± 69.30 * |
| FS-R + AP | 132.16 ± 44.50 * |
| FS-O + AP | 204.73 ± 50.85 * |
| FS-T + AP | 237.57 ± 11.99 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, M.; Rodríguez, A.; Bermúdez, E.; Roncero, E.; Andrade, M.J. Development of a Methodology for Estimating the Ergosterol in Meat Product-Borne Toxigenic Moulds to Evaluate Antifungal Agents. Foods 2021, 10, 438. https://doi.org/10.3390/foods10020438
Álvarez M, Rodríguez A, Bermúdez E, Roncero E, Andrade MJ. Development of a Methodology for Estimating the Ergosterol in Meat Product-Borne Toxigenic Moulds to Evaluate Antifungal Agents. Foods. 2021; 10(2):438. https://doi.org/10.3390/foods10020438
Chicago/Turabian StyleÁlvarez, Micaela, Alicia Rodríguez, Elena Bermúdez, Elia Roncero, and María J. Andrade. 2021. "Development of a Methodology for Estimating the Ergosterol in Meat Product-Borne Toxigenic Moulds to Evaluate Antifungal Agents" Foods 10, no. 2: 438. https://doi.org/10.3390/foods10020438
APA StyleÁlvarez, M., Rodríguez, A., Bermúdez, E., Roncero, E., & Andrade, M. J. (2021). Development of a Methodology for Estimating the Ergosterol in Meat Product-Borne Toxigenic Moulds to Evaluate Antifungal Agents. Foods, 10(2), 438. https://doi.org/10.3390/foods10020438