Multicriteria Optimization of Phenolic Compounds Capture from a Sunflower Protein Isolate Production Process by-Product by Adsorption Column and Assessment of Their Antioxidant and Anti-Inflammatory Effects
Abstract
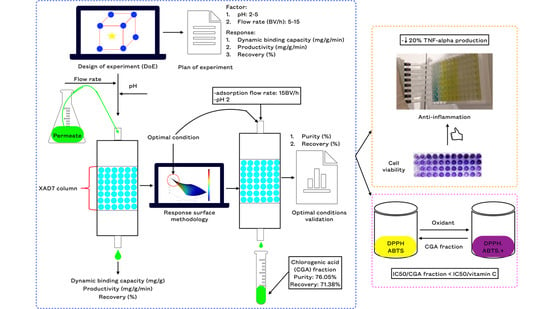
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Aqueous by-Product from Sunflower Protein Isolate Production
2.3. Column Adsorption
2.4. HPLC Analysis
2.5. Design of Experiments
2.6. Multi-Objective Optimization
2.7. Antioxidant Activity
2.7.1. DPPH Radical Scavenging Activity
2.7.2. ABTS Radical Scavenging Activity
2.8. Cell Culture and Treatments
2.9. Cell Viability
2.10. TNF-α Quantification
2.11. Data Analysis
3. Results and Discussion
3.1. Dynamic Adsorption Step
3.1.1. Effect of pH and Flow Rate on Dynamic Binding Capacity, Recovery, and Process Productivity
3.1.2. Multi-Objective Optimization
3.1.3. Dynamic Desorption Step
3.2. In Vitro Antioxidant Activity
3.3. Cytotoxicity and Anti-Inflammatory Activity of the CGA Fraction
3.3.1. Cytotoxicity
3.3.2. Anti-Inflammatory Activity of the CGA Fraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CGA | Chlorogenic acid |
| 3-CQA | 3-Caffeoylquinic acid |
| 4-CQA | 4-Caffeoylquinic acid |
| 5-CQA | 5-Caffeoylquinic acid |
| MS | Mass spectrometry |
| SFM | Sunflower meal |
| HPLC | High-performance liquid chromatography |
| DBC | Dynamic binding capacity |
| DBC10 | Dynamic binding capacity at 10% |
| DoE | Design of Experiment |
| BV | Bed volume |
| LPS | Lipopolysaccharide |
| TNF-α | Tumor necrosis factor-alpha |
| DV | Diavolume |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS | 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| IC50 | Inhibitory concentration at 50% of scavenging rate |
| RSD | Residual standard deviation |
| ANOVA | Analyze of variance |
| SD | Standard deviation |
| PMA | Phorbol myristate acetate |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| PBS | Phosphate buffer saline |
| BHA | Butyl hydroxyl anisol |
| BHT | Butylated hydroxyl toluene |
| OD | Optical density |
| Des. | Desorption |
| Ads. | Adsorption |
| UV | Ultraviolet |
| v/v | Volume/volume |
| rpm | Revolution per minute |
References
- Kremr, D.; Bajer, T.; Bajerová, P.; Surmová, S.; Ventura, K.; Kremr, D.; Bajer, T.; Bajerová, P.; Surmová, S.; Ventura, K. Unremitting Problems with Chlorogenic Acid Nomenclature: A Review. Química Nova 2016, 39, 530–533. [Google Scholar] [CrossRef]
- Park, H.J.; Davis, S.R.; Liang, H.-Y.; Rosenberg, D.W.; Bruno, R.S. Chlorogenic Acid Differentially Alters Hepatic and Small Intestinal Thiol Redox Status Without Protecting Against Azoxymethane-Induced Colon Carcinogenesis in Mice. Nutr. Cancer 2010, 62, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and Anti-Collagenase Activity of Hypericum hircinum L. Ind. Crop. Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Xi, L.; Mu, T.; Sun, H. Preparative Purification of Polyphenols from Sweet Potato (Ipomoea batatas L.) Leaves by AB-8 Macroporous Resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef]
- Huang, M.-H.; Chu, H.-L.; Juang, L.-J.; Wang, B.-S. Inhibitory Effects of Sweet Potato Leaves on Nitric Oxide Production and Protein Nitration. Food Chem. 2010, 121, 480–486. [Google Scholar] [CrossRef]
- Kurata, R.; Adachi, M.; Yamakawa, O.; Yoshimoto, M. Growth Suppression of Human Cancer Cells by Polyphenolics from Sweetpotato (Ipomoea batatas L.) Leaves. J. Agric. Food Chem. 2007, 55, 185–190. [Google Scholar] [CrossRef]
- Kurata, R.; Yahara, S.; Yamakawa, O.; Yoshimoto, M. Simple High-Yield Purification of 3,4,5-Tri-O-Caffeoylquinic Acid from Sweetpotato (Ipomoea batatas L.) Leaf and Its Inhibitory Effects on Aldose Reductase. Food Sci. Technol. Res. 2011, 17, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Shin, H.S.; Satsu, H.; Totsuka, M.; Shimizu, M. 5-Caffeoylquinic Acid and Caffeic Acid Down-Regulate the Oxidative Stress- and TNF-α-Induced Secretion of Interleukin-8 from Caco-2 Cells. J. Agric. Food Chem. 2008, 56, 3863–3868. [Google Scholar] [CrossRef]
- Shin, H.S.; Satsu, H.; Bae, M.-J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-Inflammatory Effect of Chlorogenic Acid on the IL-8 Production in Caco-2 Cells and the Dextran Sulphate Sodium-Induced Colitis Symptoms in C57BL/6 Mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic Acid Inhibits Lipopolysaccharide-Induced Cyclooxygenase-2 Expression in RAW264.7 Cells through Suppressing NF-ΚB and JNK/AP-1 Activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Costa, G.; Figueirinha, A.; Marques, C.; Pereira, P.; Miguel Neves, B.; Celeste Lopes, M.; García-Rodríguez, C.; Teresa Cruz, M.; Teresa Batista, M. Anti-Inflammatory Activity of Cymbopogon Citratus Leaves Infusion via Proteasome and Nuclear Factor-ΚB Pathway Inhibition: Contribution of Chlorogenic Acid. J. Ethnopharmacol. 2013, 148, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, Y.; Fu, C.; Li, J.; Li, Z. Simultaneous Separation and Purification of Total Polyphenols, Chlorogenic Acid and Phlorizin from Thinned Young Apples. Food Chem. 2013, 136, 1022–1029. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on Regulating Glucose and Lipids Metabolism: A Review. Evid. Based Complementary Altern. Med. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and Quantification of Phenolic Compounds from Sunflower (Helianthus annuus L.) Kernels and Shells by HPLC-DAD/ESI-MSn. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Weisz, G.M.; Carle, R.; Kammerer, D.R. Sustainable Sunflower Processing—II. Recovery of Phenolic Compounds as a by-Product of Sunflower Protein Extraction. Innov. Food Sci. Emerg. Technol. 2013, 17, 169–179. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Muzquiz, M.; García-Vallejo, C.; Burbano, C.; Cuadrado, C.; Ayet, G.; Robredo, L.M. Determination of Caffeic and Chlorogenic Acids and Their Derivatives in Different Sunflower Seeds. J. Sci. Food Agric. 2000, 80, 459–464. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 1 March 2021).
- Weisz, G.M.; Schneider, L.; Schweiggert, U.; Kammerer, D.R.; Carle, R. Sustainable Sunflower Processing—I. Development of a Process for the Adsorptive Decolorization of Sunflower [Helianthus annuus L.] Protein Extracts. Innov. Food Sci. Emerg. Technol. 2010, 11, 733–741. [Google Scholar] [CrossRef]
- Albe Slabi, S.; Mathé, C.; Framboisier, X.; Defaix, C.; Mesieres, O.; Galet, O.; Kapel, R. A New SE-HPLC Method for Simultaneous Quantification of Proteins and Main Phenolic Compounds from Sunflower Meal Aqueous Extracts. Anal. Bioanal. Chem. 2019, 411, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Dong, B.; Yuan, X.; Kuang, Q.; Zhao, Q.; Yang, M.; Liu, J.; Zhao, B. Enrichment and Separation of Chlorogenic Acid from the Extract of Eupatorium Adenophorum Spreng by Macroporous Resin. J. Chromatogr. B 2016, 1008, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.-C.; Liu, Y.; Yi, Y.-T.; Li, H.-J.; Fan, P.; Xia, C.-H. Preliminary Enrichment and Separation of Chlorogenic Acid from Helianthus tuberosus L. Leaves Extract by Macroporous Resins. Food Chem. 2015, 168, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Aymes, A.; Framboisier, X.; Ioannou, I.; Kapel, R. Adsorption of Phenolic Compounds from an Aqueous By-Product of Sunflower Protein Extraction/Purification by Macroporous Resins. J. Chromatogr. Sep. Tech. 2020, 11, 435. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 Cell Line: An in Vitro Cell Model for Immune Modulation Approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Albe Slabi, S.; Mathe, C.; Basselin, M.; Framboisier, X.; Ndiaye, M.; Galet, O.; Kapel, R. Multi-Objective Optimization of Solid/Liquid Extraction of Total Sunflower Proteins from Cold Press Meal. Food Chem. 2020, 317, 126423. [Google Scholar] [CrossRef]
- Wu, C.; Huang, M.; Lin, Y.; Ju, H.; Ching, H. Antioxidant Properties of Cortex Fraxini and Its Simple Coumarins. Food Chem. 2007, 104, 1464–1471. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, R.; Zhao, Y.; Liu, C.-Z. Separation of Chlorogenic Acid from Honeysuckle Crude Extracts by Macroporous Resins. J. Chromatogr. B 2008, 867, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lwt Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chen, C.-W.; Ho, C.-T. Antioxidant Properties of Polyphenols Extracted from Green and Black Teas. J. Food Lipids 1995, 2, 35–46. [Google Scholar] [CrossRef]
- Montoro, P.; Braca, A.; Pizza, C.; Detommasi, N. Structure-Antioxidant Activity Relationships of Flavonoids Isolated from Different Plant Species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and Cinnamic Acid Derivatives as Antioxidants: Structure−Activity Relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Liehr, M.; Mereu, A.; Pastor, J.J.; Quintela, J.C.; Staats, S.; Rimbach, G.; Ipharraguerre, I.R. Olive Oil Bioactives Protect Pigs against Experimentally-Induced Chronic Inflammation Independently of Alterations in Gut Microbiota. PLoS ONE 2017, 12, e0174239. [Google Scholar] [CrossRef] [Green Version]
- Godbout, J.P.; Glaser, R. Stress-Induced Immune Dysregulation: Implications for Wound Healing, Infectious Disease and Cancer. J. Neuroimmune Pharmacol. 2006, 1, 421–427. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]






| Regression Coefficient | Response | |||
|---|---|---|---|---|
| Y1 (Dynamic Binding Capacity, mg/g) | Y2 (Productivity, mg/g/min) | Y3 (Recovery, %) | ||
| Unscaled | βo a | 109.19 | 0.10 | −139.43 |
| β1 b | −4.31 | 0.018 | −0.68 | |
| β2 c | −29.89 | −0.05 | 147.49 | |
| β11 d | 0.085 | −0.0003 | 0.07 | |
| β22 e | 2.39 | 0.0075 | −21.53 | |
| β12 f | 0.44 | −0.0023 | −0.67 | |
| Scaled and centered | βo | 14.71 | 0.083 | 89.56 |
| β1 | −5.35 | 0.018 | −8.38 | |
| β2 | −13.11 | −0.032 | −14.85 | |
| β11 | 2.12 | −0.0080 | 1.67 | |
| β22 | 5.38 | 0.017 | −48.44 | |
| β12 | 3.30 | −0.018 | −5.003 | |
| Statistic model parameter | ||||
| R2 | 0.996 | 0.996 | 0.93 | |
| RSD g | 1.005 | 0.003 | 11.09 | |
| Regression p-value | 0.00 | 0.00 | 0.007 | |
| Lack of fit | 0.28 | 0.22 | 0.877 |
| Condition | Response Value | |
|---|---|---|
| Productivity (mg/g/min) | Recovery (%) | |
| Predicted values | 0.125 | 79.66 |
| Experimental values | 0.128 ± 0.19 | 78.77 ± 3.61 |
| EtOH Concentration (%) | Purity (%) | Recovery (ads. + des.) (%) |
|---|---|---|
| 30 | 53.29 ± 0.12 | 60.05 ± 0.32 |
| 50 | 76.05 ± 0.00 | 71.38 ± 1.59 |
| 70 | 71.89 ± 0.07 | 71.77 ± 1.71 |
| 90 | 72.31 ± 1.21 | ± 0.95 |
| Compound | IC50/DPPH (µg/mL) | IC50/ABTS (µg/mL) |
|---|---|---|
| Pure CGA (standard) | 5.76 ± 0.02a | 20.38 ± 0.02a |
| CGA fraction | 7.05 ± 0.01b | 22.52 ± 0.03b |
| Vitamin C | 7.26 ± 0.02c | 36.31 ± 0.01c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.T.; Ropars, A.; Aymes, A.; Frippiat, J.-P.; Kapel, R. Multicriteria Optimization of Phenolic Compounds Capture from a Sunflower Protein Isolate Production Process by-Product by Adsorption Column and Assessment of Their Antioxidant and Anti-Inflammatory Effects. Foods 2021, 10, 760. https://doi.org/10.3390/foods10040760
Le TT, Ropars A, Aymes A, Frippiat J-P, Kapel R. Multicriteria Optimization of Phenolic Compounds Capture from a Sunflower Protein Isolate Production Process by-Product by Adsorption Column and Assessment of Their Antioxidant and Anti-Inflammatory Effects. Foods. 2021; 10(4):760. https://doi.org/10.3390/foods10040760
Chicago/Turabian StyleLe, Tuong Thi, Armelle Ropars, Arnaud Aymes, Jean-Pol Frippiat, and Romain Kapel. 2021. "Multicriteria Optimization of Phenolic Compounds Capture from a Sunflower Protein Isolate Production Process by-Product by Adsorption Column and Assessment of Their Antioxidant and Anti-Inflammatory Effects" Foods 10, no. 4: 760. https://doi.org/10.3390/foods10040760
APA StyleLe, T. T., Ropars, A., Aymes, A., Frippiat, J. -P., & Kapel, R. (2021). Multicriteria Optimization of Phenolic Compounds Capture from a Sunflower Protein Isolate Production Process by-Product by Adsorption Column and Assessment of Their Antioxidant and Anti-Inflammatory Effects. Foods, 10(4), 760. https://doi.org/10.3390/foods10040760