Elucidation and Regulation of Polyphenols in the Smoking Process of Shanxi Aged Vinegar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Determination of TWSP and Physicochemical Indexes
2.3. Analysis of PPs Composition
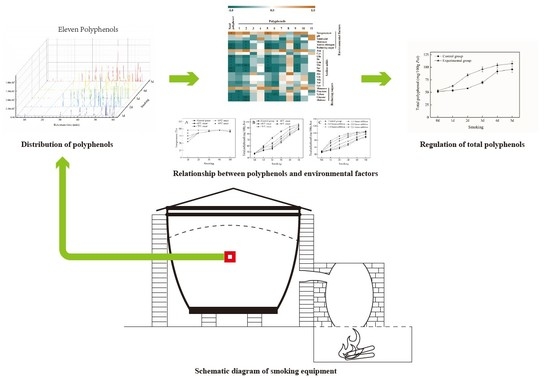
2.4. Influence of Environmental Factors on TWSP
2.5. Data Analysis Methods
3. Results
3.1. Distribution of PPs and Changes of TWSP Content during the Smoking Process
3.2. Changes in Physicochemical Indexes during the Smoking Process
3.3. Analysis of Correlation among TWSP, PPs, and Environmental Factors during the Smoking Process
3.4. Analysis of Correlation among TWSP, PPs, and Amino Acids during the Smoking Process
3.5. Analysis of Correlation among TWSP, PPs, and Reducing Sugars during the Smoking Process
3.6. Influence of Environmental Factors on TWSP
3.7. In situ Regulation of TWSP during the Smoking Process
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef] [Green Version]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, Z.; Avci, E.; Erdem, Y.K. Characterization of binding interactions between green tea flavanoids and milk proteins. Food Chem. 2010, 121, 450–456. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2020, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber-Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Phan, A.D.T.; D’Arcy, B.R.; Gidley, M.J. Polyphenol-cellulose interactions: Effects of pH, temperature and salt. Int. J. Food Sci. Technol. 2016, 51, 203–211. [Google Scholar] [CrossRef]
- Alean, J.; Chejne, F.; Rojano, B. Degradation of polyphenols during the cocoa drying process. J. Food Eng. 2016, 189, 99–105. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Colosimo, D.A.; MacMillan, J.B. Detailed Mechanistic Study of the Non-enzymatic Formation of the Discoipyrrole Family of Natural Products. J. Am. Chem. Soc. 2016, 138, 2383–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [Green Version]
- Es-Safi, N.-E.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. New phenolic compounds obtained by evolution of (+)-catechin and glyoxylic acid in hydroalcoholic medium. Tetrahedron Lett. 2000, 41, 1917–1921. [Google Scholar] [CrossRef]
- Longe, L.; Garnier, G.; Saito, K. Synthesis of Lignin-based Phenol Terminated Hyperbranched Polymer. Molecules 2019, 24, 3717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, V.K.; Gogoi, S.; Choudary, B.M.; Karak, N. A promising catalyst for exclusive para hydroxylation of substituted aromatic hydrocarbons under UV light. Green Chem. 2017, 19, 4278–4283. [Google Scholar] [CrossRef]
- Ren, M.; Wang, X.; Tian, C.; Li, X.; Zhang, B.; Song, X.; Zhang, J. Characterization of Organic Acids and Phenolic Compounds of Cereal Vinegars and Fruit Vinegars in China. J. Food Process. Preserv. 2017, 41. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Shao, Y.; Chen, F. Free Phenolic Acids in Shanxi Aged Vinegar: Changes During Aging and Synergistic Antioxidant Activities. Int. J. Food Prop. 2015, 19, 1183–1193. [Google Scholar] [CrossRef]
- Du, P.; Zhou, J.; Zhang, L.; Zhang, J.; Li, N.; Zhao, C.; Tu, L.; Zheng, Y.; Xia, T.; Luo, J.; et al. GC x GC-MS analysis and hypolipidemic effects of polyphenol extracts from Shanxi-aged vinegar in rats under a high fat diet. Food Funct. 2020. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Medina-Remon, A.; Perez-Jimenez, J.; Neveu, V.; Knaze, V.; Slimani, N.; Scalbert, A. Effects of food processing on polyphenol contents: A systematic analysis using Phenol-Explorer data. Mol. Nutr. Food Res. 2015, 59, 160–170. [Google Scholar] [CrossRef]
- Xie, X.; Zheng, Y.; Liu, X.; Cheng, C.; Zhang, X.; Xia, T.; Yu, S.; Wang, M. Antioxidant Activity of Chinese Shanxi Aged Vinegar and Its Correlation with Polyphenols and Flavonoids during the Brewing Process. J. Food Sci. 2017, 82, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Zheng, Y.; Wang, M.; Han, Y.; Wang, Y.; Luo, J.; Niu, D. Exploring microbial succession and diversity during solid-state fermentation of Tianjin duliu mature vinegar. Bioresour. Technol. 2013, 148, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jia, Y.X.; Su, Y.; Zhang, X.Y.; Tu, L.N.; Nie, Z.Q.; Zheng, Y.; Wang, M. Initial Analysis on the Characteristics and Synthesis of Exopolysaccharides from Sclerotium rolfsii with Different Sugars as Carbon Sources. Polymer (Basel) 2020, 12, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekedam, E.K.; Loots, M.J.; Schols, H.A.; Van Boekel, M.A.; Smit, G. Roasting effects on formation mechanisms of coffee brew melanoidins. J. Agric. Food Chem. 2008, 56, 7138–7145. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2016, 20, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Cui, H.; Zhang, Q.; Hayat, K.; Zhan, H.; Yu, J.; Jia, C.; Zhang, X.; Ho, C.T. Adducts Derived from (-)-Epigallocatechin gallate-Amadori Rearrangement Product in Aqueous Reaction System: Characterization, Formation and Thermolysis. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef]
- Ramamurthy, T.V.; Ravi, S.; Viswanathan, K.V. An improved synthesis of Carbon-14 labelled carboxylic acids from Carbon-14 labelled amino acids. J. Label. Compd. Radiopharmaceut. 1988, 25, 809–814. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Sun, L.J.; Wang, X.F. Synthesis of p-Hydroxyphenylacetic Acid. Chem. World 2007, 06, 360–361. [Google Scholar] [CrossRef]
- van Schijndel, J.; Molendijk, D.; van Beurden, K.; Canalle, L.A.; Noël, T.; Meuldijk, J. Preparation of bio-based styrene alternatives and their free radical polymerization. Eur. Polym. J. 2020, 125. [Google Scholar] [CrossRef]
- Assal, M.; Shaik, M.; Kuniyil, M.; Khan, M.; Alzahrani, A.; Al-Warthan, A.; Siddiqui, M.; Adil, S. Mixed Zinc/Manganese on Highly Reduced Graphene Oxide: A Highly Active Nanocomposite Catalyst for Aerial Oxidation of Benzylic Alcohols. Catalysts 2017, 7, 391. [Google Scholar] [CrossRef] [Green Version]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2015, 4, 35–46. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, K. Anti-Plant Virus Agent of Ferulic Acid and deRivatives Thereof. WO Patent 2011069444, 12 July 2010. [Google Scholar]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Hedegaard, R.V.; Skibsted, L.H.; Andersen, M.L. Epicatechin and epigallocatechin gallate inhibit formation of intermediary radicals during heating of lysine and glucose. Food Chem. 2014, 146, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Bors, W.; Stettmaier, K. Radical-assisted melanoidin formation during thermal processing of foods as well as under physiological conditions. J. Agric. Food Chem. 1999, 47, 391–396. [Google Scholar] [CrossRef]
- Lin, Q.; Han, L.; Liu, G.; Cheng, W.; Wang, L. A preliminary study on the formation pathways of glycated phosphatidylethanolamine of food rich in phospholipid during the heat-processing. Rsc Adv. 2018, 8, 11280–11288. [Google Scholar] [CrossRef] [Green Version]
- Taran, F.; Renard, P.Y.; Bernard, H.; Mioskowski, C.; Frobert, Y.; Pradelles, P.; Grassi, J. Antibody-Catalyzed Decarboxylative Oxidation of Vanillylmandelic Acid. J. Am. Chem. Soc. 1998, 120, 3332–3339. [Google Scholar] [CrossRef]
- Bjørsvik, H.-R.; Liguori, L.; Minisci, F. High Selectivity in the Oxidation of Mandelic Acid Derivatives and inO-Methylation of Protocatechualdehyde: New Processes for Synthesis of Vanillin, iso-Vanillin, and Heliotropin. Org. Process Res. Dev. 2000, 4, 534–543. [Google Scholar] [CrossRef]






| Polyphenols | CAS Number | Compound Identification | Smoking | ||||
|---|---|---|---|---|---|---|---|
| 1 Day | 2 Days | 3 Days | 4 Days | 5 Days | |||
| 1 | 121-33-5 | 4-Hydroxy-3-methoxy-benzaldehyde (Vanillin) | 1.95 ± 0.19 | 1.04 ± 0.11 | 3.23 ± 0.13 | 2.70 ± 0.31 | 5.67 ± 0.14 |
| 2 | 121-34-6 | 4-Hydroxy-3-methoxy-benzoic acid (Vanillic acid) | 3.65 ± 0.17 | 3.50 ± 0.22 | 5.44 ± 0.08 | 6.98 ± 0.19 | 10.43 ± 0.17 |
| 3 | 537-98-4 | (2E)-3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid ((E)-ferulicacid) | 7.30 ± 0.36 | 13.07 ± 0.11 | 6.26 ± 0.23 | 11.37 ± 0.12 | 29.57 ± 0.16 |
| 4 | 501-98-4 | (E)-3-(4-hydroxyphenyl)-2-propenoicacid ((E)-4-hydroxycinnamic acid) | 4.55 ± 0.51 | 9.19 ± 0.04 | 10.44 ± 0.073 | 15.00 ± 0.30 | 27.01 ± 0.11 |
| 5 | 99-96-7 | 4-Hydroxybenzoic acid | 0 | 0.40 ± 0.13 | 0.78 ± 0.31 | 0.91 ± 0.06 | 1.26 ± 0.01 |
| 6 | 93376-04-6 | (3R,4R)-Dihydro-3,4-bis[(3-hydroxy-4-methoxyphenyl) methyl]-2(3H)-furanone | 0 | 0 | 13.14 ± 0.14 | 11.74 ± 0.12 | 19.99 ± 0.14 |
| 7 | 55-10-7 | 4-Hydroxy-3-methoxymandelic acid (Vanillinmandelic acid) | 0.57 ± 0.18 | 0 | 0 | 1.18 ± 0.18 | 2.07 ± 0.07 |
| 8 | 40979-91-7 | 1-(3-Hydroxy-4-methoxyphenyl)-1,2-ethanediol | 0.37 ± 0.19 | 10.85 ± 0.17 | 0 | 0 | 3.73 ± 0.21 |
| 9 | 99-10-5 | 3,5-Dihydroxybenzoic acid | 0 | 0 | 0 | 5.58 ± 0.25 | 12.69 ± 0.31 |
| 10 | 96251-92-2 | (E)-3-(3-hydroxyphenyl) acrylic acid ethyl ester | 0 | 0 | 0 | 7.03 ± 0.15 | 0 |
| 11 | 1135-23-5 | 3-(4-Hydroxy-3-methoxyphenyl) propionic acid (Dihydroferulic acid) | 0 | 0 | 1.03 ± 0.15 | 0 | 0 |
| Name | Smoking | ||||
|---|---|---|---|---|---|
| 1 Day | 2 Days | 3 Days | 4 Days | 5 Days | |
| Total water-soluble polyphenols (mg/100 g Pei) | 53.60 ± 1.57 | 57.78 ± 1.01 | 69.47 ± 2.93 | 91.59 ± 5.00 | 95.45 ± 5.48 |
| Polyphenols | CAS Number | Compound Identification | Smoking | ||||
|---|---|---|---|---|---|---|---|
| 1 Day | 2 Days | 3 Days | 4 Days | 5 Days | |||
| 1 | 121-33-5 | 4-Hydroxy-3-methoxy-benzaldehyde (Vanillin) | 2.34 ± 0.21 | 2.58 ± 0.16 | 4.34 ± 0.18 | 4.62 ± 0.26 | 6.82 ± 0.25 |
| 2 | 121-34-6 | 4-Hydroxy-3-methoxy-benzoic acid (Vanillic acid) | 3.15 ± 0.26 | 4.24 ± 0.17 | 6.47 ± 0.28 | 9.78 ± 0.39 | 14.14 ± 0.47 |
| 3 | 537-98-4 | (2E)-3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid ((E)-ferulicacid) | 7.77 ± 0.39 | 16.27 ± 0.22 | 29.23 ± 0.29 | 32.31 ± 0.42 | 37.52 ± 0.53 |
| 4 | 501-98-4 | (E)-3-(4-hydroxyphenyl)-2-propenoicacid ((E)-4-hydroxycinnamic acid) | 3.23 ± 0.18 | 5.19 ± 0.24 | 6.62 ± 0.14 | 7.43 ± 0.36 | 7.33 ± 0.32 |
| 5 | 99-96-7 | 4-Hydroxybenzoic acid | 0.66 ± 0.21 | 0.74 ± 0.16 | 0.83 ± 0.25 | 1.21 ± 0.32 | 1.76 ± 0.22 |
| 6 | 93376-04-6 | (3R,4R)-Dihydro-3,4-bis[(3-hydroxy-4-methoxyphenyl) methyl]-2(3H)-furanone | 0 | 7.78 ± 0.31 | 11.27 ± 0.19 | 22.14 ± 0.33 | 23.28 ± 0.43 |
| 7 | 55-10-7 | 4-Hydroxy-3-methoxymandelic acid (Vanillinmandelic acid) | 0 | 0 | 0 | 0 | 1.39 ± 0.12 |
| 8 | 40979-91-7 | 1-(3-Hydroxy-4-methoxyphenyl)-1,2-ethanediol | 1.26 ± 0.14 | 0.18 ± 0.14 | 0 | 0 | 0 |
| 9 | 99-10-5 | 3,5-Dihydroxybenzoic acid | 3.11 ± 0.17 | 3.88 ± 0.26 | 3.61 ± 0.13 | 4.44 ± 0.32 | 7.39 ± 0.22 |
| 10 | 96251-92-2 | (E)-3-(3-hydroxyphenyl) acrylic acid ethyl ester | 0 | 0 | 0 | 1.15 ± 0.17 | 1.07 ± 0.14 |
| 11 | 1135-23-5 | 3-(4-Hydroxy-3-methoxyphenyl) propionic acid (Dihydroferulic acid) | 3.24 ± 0.16 | 6.26 ± 0.33 | 5.44 ± 0.36 | 0 | 0 |
| 12 | 102-32-9 | 3,4-Dihydroxybenzeneacetic acid (Homoprotocatechuic acid) | 9.02 ± 0.20 | 0 | 0 | 0 | 0 |
| 13 | 154-23-4 | (2R,3S)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol (Catechin) | 0.19 ± 0.04 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Song, J.; Fan, B.; Li, X.; Li, Y.; Mou, F.; Zheng, Y.; Wang, M. Elucidation and Regulation of Polyphenols in the Smoking Process of Shanxi Aged Vinegar. Foods 2021, 10, 1518. https://doi.org/10.3390/foods10071518
Xie S, Song J, Fan B, Li X, Li Y, Mou F, Zheng Y, Wang M. Elucidation and Regulation of Polyphenols in the Smoking Process of Shanxi Aged Vinegar. Foods. 2021; 10(7):1518. https://doi.org/10.3390/foods10071518
Chicago/Turabian StyleXie, Sankuan, Jia Song, Bingqian Fan, Xuan Li, Yingqi Li, Fangming Mou, Yu Zheng, and Min Wang. 2021. "Elucidation and Regulation of Polyphenols in the Smoking Process of Shanxi Aged Vinegar" Foods 10, no. 7: 1518. https://doi.org/10.3390/foods10071518
APA StyleXie, S., Song, J., Fan, B., Li, X., Li, Y., Mou, F., Zheng, Y., & Wang, M. (2021). Elucidation and Regulation of Polyphenols in the Smoking Process of Shanxi Aged Vinegar. Foods, 10(7), 1518. https://doi.org/10.3390/foods10071518