Improved Performance of D-Psicose 3-Epimerase by Immobilisation on Amino-Epoxide Support with Intense Multipoint Attachment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of DPEase
2.3. Enzyme Activitity and Protein Concentration Assay
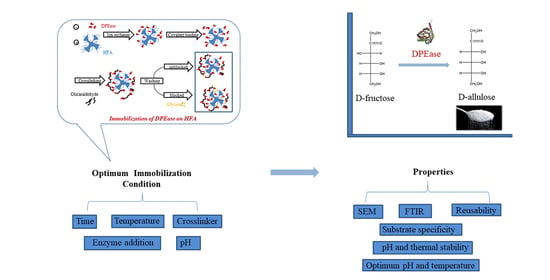
2.4. Immobilisation
2.4.1. Immobilisation Method
2.4.2. Effect of Immobilisation Conditions on Enzyme Activities and Protein Loading Efficiency
2.5. Characterization of Immobilised DPEase
2.6. Properties of Free and Immobilised DPEase
2.6.1. Optimum pH and Temperature
2.6.2. pH and thermal stability
2.6.3. Substrate Specificity
2.6.4. Reusability
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Conditions on Immobilisation
3.2. Characterisation of Immobilised DPEase
3.3. Optimum pH and Temperature of Free and Immobilised DPEase
3.4. pH and Thermal Stability of Free And immobilised DPEase
3.5. Substrate Specificity
3.6. Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matsuo, T.; Suzuki, H.; Hashiguchi, M.; Izumori, K. D-psicose is a rare sugar that provides no energy to growing rats. J. Nutr. Sci. Vitaminol. 2002, 48, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Oshima, H.; Kimura, I.; Izumori, K. Psicose contents in various food products and its origin. Food Sci. Technol. Res. 2006, 12, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Mu, W.; Chu, F.; Zhang, X.; Jiang, B.; Zhou, L.; Zhang, T. A D-psicose 3-epimerase with neutral pH optimum from Clostridium bolteae for D-psicose production: Cloning, expression, purification, and characterization. Appl. Microbiol. Biotechnol. 2014, 98, 717–725. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, Y.; Wang, J.; Huang, H.; Geng, X.; Tong, Y.; Wang, Z. Preparation of a flower-like immobilized D-psicose 3-epimerase with enhanced catalytic performance. Catalysts 2018, 8, 468. [Google Scholar] [CrossRef] [Green Version]
- Izumori, K. Izumoring: A strategy for bioproduction of all hexoses. J. Biotechnol. 2006, 124, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2012, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Busto, M.D.; Ramos-Gomez, S.; Palacios, D.; Pilar-Izquierdo, M.C.; Ortega, N. Encapsulation of glucose oxidase in alginate hollow beads to reduce the fermentable sugars in simulated musts. Food Biosci. 2018, 24, 67–72. [Google Scholar] [CrossRef]
- Itoh, H.; Sato, T.; Izumori, K. Preparation of D-psicose from D-fructose by immobilized D-tagatose 3-epimerase. J. Ferment. Bioeng. 1995, 80, 184–185. [Google Scholar] [CrossRef]
- Takeshita, K.; Suga, A.; Takada, G.; Izumori, K. Mass production of D-psicose from D-fructose by a continuous bioreactor system using immobilized D-tagatose 3-epimerase. J. Biosci. Bioeng. 2000, 90, 453–455. [Google Scholar] [CrossRef]
- Lim, B.-C.; Kim, H.-J.; Oh, D.-K. A stable immobilised D-psicose 3-epimerase for the production of D-psicose in the presence of borate. Process Biochem. 2009, 44, 822–828. [Google Scholar] [CrossRef]
- Li, C.; Lin, J.; Guo, Q.; Zhang, C.; Du, K.; Lin, H.; Lin, J. D-psicose 3-epimerase secretory overexpression, immobilization, and D-psicose biotransformation, separation and crystallization. J. Chem. Technol. Biotechnol. 2018, 93, 350–357. [Google Scholar] [CrossRef]
- He, W.; Jiang, B.; Mu, W.; Zhang, T. Production of D-allulose with D-psicose 3-epimerase expressed and displayed on the surface of Bacillus subtilis spores. J. Agric. Food Chem. 2016, 64, 7201–7207. [Google Scholar] [CrossRef]
- Ran, G.; Tan, D.; Zhao, J.; Fan, F.; Zhang, Q.; Wu, X.; Fan, P.; Fang, X.; Lu, X. Functionalized polyhydroxyalkanoate nano-beads as a stable biocatalyst for cost-effective production of the rare sugar D-allulose. Bioresour. Technol. 2019, 289, 121673. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Fernández-Lorente, G.; Abian, O.; Fernández-Lafuente, R.; Guisán, J.M. Multifunctional epoxy supports: A new tool to improve the covalent immobilization of proteins. The promotion of physical adsorptions of proteins on the supports before their covalent linkage. Biomacromolecules 2000, 1, 739–745. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernández-Lorente, G.; Pedroche, J.; Fernández-Lafuente, R.; Guisan, J.M. Epoxy sepabeads: A novel epoxy support for stabilization of industrial enzymes via very intense multipoint covalent attachment. Biotechnol. Prog. 2002, 18, 629–634. [Google Scholar] [CrossRef]
- Mateo, C.; Grazu, V.; Pessela, B.C.C.; Montes, T.; Palomo, J.M.; Torres, R.; Lopez-Gallego, F.; Fernandez-Lafuente, R.; Guisan, J.M. Advances in the design of new epoxy supports for enzyme immobilization-stabilization. Biochem. Soc. Trans. 2007, 35, 1593–1601. [Google Scholar] [CrossRef] [Green Version]
- Ummirul, K.; Mohd, S.; Kok-Gan, C.; Kian, G. Immobilization of α-Amylase from Anoxybacillus sp. SK3-4 on ReliZyme and Immobead supports. Molecules 2016, 21, 1196. [Google Scholar]
- Torres, R.; Mateo, C.; Fernández-Lorente, G.; Ortiz, C.; Fuentes, M.; Palomo, J.M.; Guisan, J.M.; Fernández-Lafuente, R. A novel heterofunctional epoxy-amino sepabeads for a new enzyme immobilisation protocol: Immobilization-stabilization of β-galactosidase from Aspergillus oryzae. Biotechnol. Prog. 2003, 19, 1056–1060. [Google Scholar] [CrossRef]
- Cerdobbel, A.; Desmet, T.; Winter, K.D.; Maertens, J.; Soetaert, W. Increasing the thermostability of sucrose phosphorylase by multipoint covalent immobilization. J. Biotechnol. 2010, 150, 125–130. [Google Scholar] [CrossRef]
- Konst, P.M.; Franssen, M.C.R.; Scott, E.L.; Sanders, J.P.M. A study on the applicability of L-aspartate α-decarboxylase in the biobased production of nitrogen containing chemicals. Green Chem. 2009, 11, 1646–1652. [Google Scholar] [CrossRef]
- He, W.; Mu, W.; Jiang, B.; Yan, X.; Zhang, T. Construction of a food grade recombinant Bacillus subtilis based on replicative plasmids with an auxotrophic marker for biotransformation of D-Fructose to D-Allulose. J. Agric. Food Chem. 2016, 64, 3243–3250. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2013, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Hoarau, M.; Badieyan, S.; Marsh, E.N.G. Immobilized enzymes: Understanding enzyme–surface interactions at the molecular level. Org. Biomol. Chem. 2017, 15, 9539–9551. [Google Scholar] [CrossRef]
- Turková, J.; Bláha, K.; Malaníková, M.; Vancurová, D.; Kálal, J. Methacrylate gels with epoxide groups as supports for immobilization of enzymes in pH range 3-12. Biochim. Biophys. Acta 1978, 524, 162–169. [Google Scholar] [CrossRef]
- Katchalski-Katzir, E.; Kraemer, D.M. Eupergit C, a carrier for immobilization of enzymes of industrial potential. J. Mol. Catal. B Enzym. 2000, 10, 157–176. [Google Scholar] [CrossRef]
- Okuda, K.; Urabe, I.; Yamada, Y.; Okada, H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991, 71, 100–105. [Google Scholar] [CrossRef]
- Hormigo, D.; de la Mata, I.; Acebal, C.; Arroyo, M. Immobilized aculeacin A acylase from Actinoplanes utahensis: Characterization of a novel biocatalyst. Bioresour. Technol. 2010, 101, 4261–4268. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Batista-Viera, F. Improved biocatalysts based on Bacillus circulans β-galactosidase immobilized onto epoxy-activated acrylic supports: Applications in whey processing. J. Mol. Catal. B-Enzym. 2012, 83, 57–64. [Google Scholar] [CrossRef]
- Mendes, A.A.; Castro, H.F.D.; Andrade, G.S.S.; Tardioli, P.W.; Giordano, R.D.L.C. Preparation and application of epoxy–chitosan/alginate support in the immobilization of microbial lipases by covalent attachment. React. Funct. Polym. 2013, 73, 160–167. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef]
- Monier, M.; El-Sokkary, A.; Sarhan, A. Immobilization of Candida rugosa lipase on modified natural wool fibers. React. Funct. Polym. 2010, 70, 122–128. [Google Scholar] [CrossRef]
- Kahar, U.M.; Chan, K.G.; Sani, M.H.; Mohd Noh, N.I.; Goh, K.M. Effects of single and co-immobilization on the product specificity of type I pullulanase from Anoxybacillus sp. SK3-4. Int. J. Biol. Macromol. 2017, 104, 322–332. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, S.; Zhang, T.; Jiang, B.; Mu, W. Recent advances in D-allulose: Physiological functionalities, applications, and biological production. Trends Food Sci. Technol. 2016, 54, 127–137. [Google Scholar] [CrossRef]
- Kim, H.J.; Lim, B.C.; Yeom, S.J.; Kim, Y.S.; Kim, D.; Oh, D.K. Roles of Ile66 and Ala107 of D-psicose 3-epimerase from Agrobacterium tumefaciens in binding O6 of its substrate, D-fructose. Biotechnol. Lett. 2010, 32, 113–118. [Google Scholar] [CrossRef]
- Pyykkö, P.; Atsumi, M. Molecular double-bond covalent radii for elements Li-E112. Chem. Eur. J. 2009, 15, 12770–12779. [Google Scholar] [CrossRef] [PubMed]









| Treatment | Optimum Immobilisation Condition | Immobilisation Results | |||||
|---|---|---|---|---|---|---|---|
| Applied Enzyme Loads (U/g Dry Resin) | Time (h) | Temperature (°C) | pH | Enzyme Activity (U/g Dry Resin) | Activity Recovery (%) | Protein Loading Efficiency (%) | |
| Ion exchange | 200 | 8 | 20 | 7.5 | 65.6 ± 4.0 D | 32.8± 2.0 D | 58.2 ± 2.2 C |
| Ion exchange + Covalent binding | 200 | 8 + 12 | 20 | 7.5 | 89.2 ± 3.2 C | 44.6 ± 1.6 C | 77.5 ± 3.1 B |
| Ion exchange + Covalent binding + Crosslinking | 200 | 8 + 12 + 1 | 20 | 7.5 | 138.8± 2.6 A | 69.4 ± 1.3 A | 90.6 ± 2.0 A |
| Ion exchange + Covalent binding + Crosslinking + Blocking | 200 | 8 + 12 + 1 + 16 | 20 | 7.5 | 103.5 ± 2.6 B | 51.8 ± 1.3 B | 90.6 ± 2.0 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Y.; Zhang, T.; Jiang, B.; Chen, J. Improved Performance of D-Psicose 3-Epimerase by Immobilisation on Amino-Epoxide Support with Intense Multipoint Attachment. Foods 2021, 10, 831. https://doi.org/10.3390/foods10040831
Bu Y, Zhang T, Jiang B, Chen J. Improved Performance of D-Psicose 3-Epimerase by Immobilisation on Amino-Epoxide Support with Intense Multipoint Attachment. Foods. 2021; 10(4):831. https://doi.org/10.3390/foods10040831
Chicago/Turabian StyleBu, Yifan, Tao Zhang, Bo Jiang, and Jingjing Chen. 2021. "Improved Performance of D-Psicose 3-Epimerase by Immobilisation on Amino-Epoxide Support with Intense Multipoint Attachment" Foods 10, no. 4: 831. https://doi.org/10.3390/foods10040831
APA StyleBu, Y., Zhang, T., Jiang, B., & Chen, J. (2021). Improved Performance of D-Psicose 3-Epimerase by Immobilisation on Amino-Epoxide Support with Intense Multipoint Attachment. Foods, 10(4), 831. https://doi.org/10.3390/foods10040831