Influence of Glycomacropeptide on Rehydration Characteristics of Micellar Casein Concentrate Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
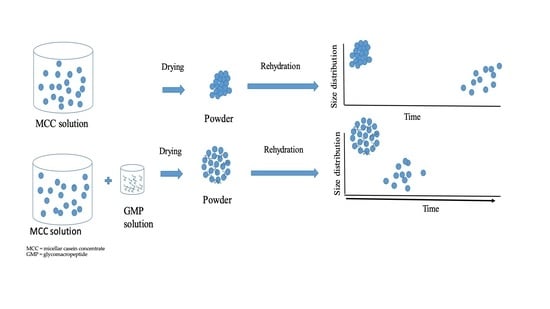
2.2. Preparation of Micellar Casein Concentrate and Glycomacropeptide Solutions and Powders
2.3. Compositional Analysis of Protein Solutions and Powders
2.4. Particle Size and Charge in Protein Solutions
2.5. Morphology of Powders
2.6. Rehydration Properties of Powders
2.6.1. Contact Angle of Powder Samples
2.6.2. Dispersion Characteristics of Powders
2.7. Protein Release in Solution during Powder Rehydration
2.8. Data Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Protein Solutions
3.2. Composition and Microstructure of Protein Powders
3.3. Wettability of Micellar Casein Concentrate Powders
3.4. Kinetics of Powder Dispersion
3.5. Casein and Glycomacropeptide Solubilization in Aqueous Phase during Powder Dispersion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agarwal, S.; Beausire, R.L.; Patel, S.; Patel, H. Innovative uses of milk protein concentrates in product development. J. Food Sci. 2015, 80 (Suppl. 1), A23–A29. [Google Scholar] [CrossRef]
- Felix da Silva, D.; Ahrné, L.; Ipsen, R.; Hougaard, A.B. Casein-based powders: Characteristics and rehydration properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 240–254. [Google Scholar] [CrossRef] [Green Version]
- Crowley, S.V.; Kelly, A.L.; Schuck, P.; Jeantet, R.; O’Mahony, J.A. Rehydration and solubility characteristics of high-protein dairy powders. In Advanced Dairy Chemistry; McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer: New York, NY, USA, 2016; pp. 99–131. [Google Scholar]
- Mimouni, A.; Deeth, H.C.; Whittaker, A.K.; Gidley, M.J.; Bhandari, B.R. Rehydration process of milk protein concentrate powder monitored by static light scattering. Food Hydrocoll. 2009, 23, 1958–1965. [Google Scholar] [CrossRef]
- Maidannyk, V.; Lutjes, E.; Montgomery, S.; McCarthy, N.; Auty, M.A.E. Measurement of effective diffusion coefficients in dairy powders by confocal microscopy and sorption kinetic profiles. Food Struct. 2019, 20, 100108. [Google Scholar] [CrossRef]
- Schuck, P.; Mejean, S.; Dolivet, A.; Gaiani, C.; Banon, S.; Scher, J.; Jeantet, R. Water transfer during rehydration of micellar casein powders. Lait 2007, 87, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Mimouni, A.; Deeth, H.C.; Whittaker, A.K.; Gidley, M.J.; Bhandari, B.R. Investigation of the microstructure of milk protein concentrate powders during rehydration: Alterations during storage. J. Dairy Sci. 2010, 93, 463–472. [Google Scholar] [CrossRef]
- Fyfe, K.N.; Kravchuk, O.; Le, T.; Deeth, H.C.; Nguyen, A.V.; Bhandari, B.R. Storage induced changes to high protein powders: Influence on surface properties and solubility. J. Sci. Food Agric. 2011, 91, 2566–2575. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef]
- Anema, S.G.; Pinder, D.N.; Hunter, R.J.; Hemar, Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85). Food Hydrocoll. 2006, 20, 386–393. [Google Scholar] [CrossRef]
- Bansal, N.; Truong, T.; Bhandari, B. Feasibility study of lecithin nanovesicles as spacers to improve the solubility of milk protein concentrate powder during storage. Dairy Sci. Technol. 2017, 96, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Schokker, E.P.; Church, J.S.; Mata, J.P.; Gilbert, E.P.; Puvanenthiran, A.; Udabage, P. Reconstitution properties of micellar casein powder: Effects of composition and storage. Int. Dairy J. 2011, 21, 877–886. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Li, H.; Li, S.; Mo, B.; Lv, J. Functionality of milk protein concentrate 80 with emulsifying salts and its applications in analogue cheeses. Int. J. Food Prop. 2016, 20, 2594–2607. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Power, O.; Wijayanti, H.B.; Kelly, P.M.; Mao, L.; Fenelon, M.A. Effects of calcium chelating agents on the solubility of milk protein concentrate. Int. J. Dairy Tech. 2017, 70, 415–423. [Google Scholar] [CrossRef]
- Power, O.M.; Fenelon, M.A.; O’Mahony, J.A.; McCarthy, N.A. Influence of sodium hexametaphosphate addition on the functional properties of milk protein concentrate solutions containing transglutaminase cross-linked proteins. Int. Dairy J. 2020, 104, 104641. [Google Scholar] [CrossRef]
- Sharma, R.; Rajput, Y.S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Technol. 2013, 93, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Manso, M.A.; Lopez-Fandio, R. κ-Casein macropeptides from cheese whey: Physicochemical, biological, nutritional, and technological features for possible uses. Food Rev. Int. 2004, 20, 329–355. [Google Scholar] [CrossRef]
- Croguennec, T.; Leng, N.; Hamon, P.; Rousseau, F.; Jeantet, R.; Bouhallab, S. Caseinomacropeptide modifies the heat-induced denaturation–aggregation process of β-lactoglobulin. Int. Dairy J. 2014, 36, 55–64. [Google Scholar] [CrossRef]
- Farias, M.E.; Martinez, M.J.; Pilosof, A.M.R. Casein glycomacropeptide pH-dependent self-assembly and cold gelation. Int. Dairy J. 2010, 20, 79–88. [Google Scholar] [CrossRef]
- Martinez, M.J.; Pizones Ruiz-Henestrosa, V.M.; Carrera Sanchez, C.; Rodriguez Patino, J.M.; Pilosof, A.M.R. Foaming and surface properties of casein glycomacropeptide-gelatin mixtures as affected by their interactions in the aqueous phase. Food Hydrocoll. 2013, 33, 48–57. [Google Scholar] [CrossRef]
- Morales, R.; Martinez, M.J.; Pilosof, A.M.R. Synergistic effect of casein glycomacropeptide on sodium caseinate foaming properties. Colloids Surf. B. 2017, 159, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, R.; Martinez, M.J.; Pilosof, A.M.R. Impact of casein glycomacropeptide on sodium caseinate self-assembly and gelation. Int. Dairy J. 2015, 49, 30–36. [Google Scholar] [CrossRef]
- Loria, K.G.; Pilosof, A.M.R.; Farías, M.E. Influence of calcium and sodium chloride on caseinomacropeptide self-assembly and flow behaviour at neutral pH. LWT 2018, 98, 598–605. [Google Scholar] [CrossRef]
- Jaques, L.W.; Brown, E.B.; Barrett, J.M.; Brey, W.S.; Weltner, W. Sialic-acid—A calcium-binding carbohydrate. J. Biol. Chem. 1977, 252, 4533–4538. [Google Scholar] [CrossRef]
- ISO. Milk—Determination of Nitrogen Content; ISO 8969-2. IDF Standard 20-2; International Dairy Federation: Brussels, Belgium, 2001. [Google Scholar]
- IDF. Dried Milk and Dried Cream—Determination of Water Content; Standard No. 5537; International Dairy Federation: Brussels, Belgium, 1993. [Google Scholar]
- Poste, G.; Moss, C. The study of surface reactions in biological systems by ellipsometry. Prog. Surf. Sci. 1972, 2, 139–232. [Google Scholar] [CrossRef]
- Crowley, S.V.; Desautel, B.; Gazi, I.; Kelly, A.L.; Huppertz, T.; O’Mahony, J.A. Rehydration characteristics of milk protein concentrate powders. J. Food Eng. 2015, 149, 105–113. [Google Scholar] [CrossRef]
- Panthi, R.R.; Bot, F.; Shibu, S.N.; Saladukha, D.; Ochalski, T.J.; O’Mahony, J.A. Influence of pH adjustment on physicochemical properties of microfiltration retentates of skim milk and rehydration properties of resulting powders. Int. Dairy J. 2021, 116. [Google Scholar] [CrossRef]
- Wu, S.; Fitzpatrick, J.; Cronin, K.; Miao, S. The effect of pH on the wetting and dissolution of milk protein isolate powder. J. Food Eng. 2019, 240, 114–119. [Google Scholar] [CrossRef]
- Pathania, S.; Ho, Q.T.; Hogan, S.A.; McCarthy, N.; Tobin, J.T. Applications of hydrodynamic cavitation for instant rehydration of high protein milk powders. J. Food Eng. 2018, 225, 18–25. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Kelly, P.M.; Maher, P.G.; Fenelon, M.A. Dissolution of milk protein concentrate (MPC) powders by ultrasonication. J. Food Eng. 2014, 126, 142–148. [Google Scholar] [CrossRef]
- Bouvier, J.-M.; Collado, M.; Gardiner, D.; Scott, M.; Schuck, P. Physical and rehydration properties of milk protein concentrates: Comparison of spray-dried and extrusion-porosified powders. Dairy Sci. Technol. 2013, 93, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Udabage, P.; Puvanenthiran, A.; Yoo, J.A.; Versteeg, C.; Augustin, M.A. Modified water solubility of milk protein concentrate powders through the application of static high pressure treatment. J. Dairy Res. 2012, 79, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Bot, F.; Crowley, S.V.; O’Mahony, J.A. Solubility enhancement of milk protein isolate by sodium caseinate addition: Comparison between wet- and dry-blending approaches. Int. Dairy J. 2020, 105, 104661. [Google Scholar] [CrossRef]
- Mimouni, A.; Deeth, H.C.; Whittaker, A.K.; Gidley, M.J.; Bhandari, B.R. Rehydration of high-protein-containing dairy powder: Slow- and fast-dissolving components and storage effects. Dairy Sci. Tech. 2010, 90, 335–344. [Google Scholar] [CrossRef]
- Gazi, I.; Huppertz, T. Influence of protein content and storage conditions on the solubility of caseins and whey proteins in milk protein concentrates. Int. Dairy J. 2015, 46, 22–30. [Google Scholar] [CrossRef]



 ), κ (
), κ (  ), α (
), α (  ), and β (
), and β (  ) caseins in solution at 25 °C as a function of rehydration time. (A) Dispersion of powder containing no GMP (P-MCC-0G), (B) dispersion of powder containing 10% GMP (P-MCC-10G), or (C) 20% GMP (P-MCC-20G) as a percentage of total protein.
) caseins in solution at 25 °C as a function of rehydration time. (A) Dispersion of powder containing no GMP (P-MCC-0G), (B) dispersion of powder containing 10% GMP (P-MCC-10G), or (C) 20% GMP (P-MCC-20G) as a percentage of total protein.
 ), κ (
), κ (  ), α (
), α (  ), and β (
), and β (  ) caseins in solution at 25 °C as a function of rehydration time. (A) Dispersion of powder containing no GMP (P-MCC-0G), (B) dispersion of powder containing 10% GMP (P-MCC-10G), or (C) 20% GMP (P-MCC-20G) as a percentage of total protein.
) caseins in solution at 25 °C as a function of rehydration time. (A) Dispersion of powder containing no GMP (P-MCC-0G), (B) dispersion of powder containing 10% GMP (P-MCC-10G), or (C) 20% GMP (P-MCC-20G) as a percentage of total protein.
| Parameter | S-MCC-0G | S-MCC-10G | S-MCC-20G |
|---|---|---|---|
| Total solids (%, w/w) | 9.77 ± 0.11 a | 9.90 ± 0.13 a | 9.92 ± 0.14 a |
| Protein (%, w/w) | 8.64 ± 0.08 a | 8.58 ± 0.09 a | 8.59 ± 0.18 a |
| Protein (%, w/w, dry basis) | 88.5 ± 0.72 a | 86.7 ± 0.31 a | 86.6 ± 1.21 a |
| Zeta potential (mV) | −25.8 ± 1.37 b | −29.6 ± 1.97 ab | −31.6 ± 1.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panthi, R.R.; Bot, F.; O’Mahony, J.A. Influence of Glycomacropeptide on Rehydration Characteristics of Micellar Casein Concentrate Powder. Foods 2021, 10, 1960. https://doi.org/10.3390/foods10081960
Panthi RR, Bot F, O’Mahony JA. Influence of Glycomacropeptide on Rehydration Characteristics of Micellar Casein Concentrate Powder. Foods. 2021; 10(8):1960. https://doi.org/10.3390/foods10081960
Chicago/Turabian StylePanthi, Ram R., Francesca Bot, and James A. O’Mahony. 2021. "Influence of Glycomacropeptide on Rehydration Characteristics of Micellar Casein Concentrate Powder" Foods 10, no. 8: 1960. https://doi.org/10.3390/foods10081960
APA StylePanthi, R. R., Bot, F., & O’Mahony, J. A. (2021). Influence of Glycomacropeptide on Rehydration Characteristics of Micellar Casein Concentrate Powder. Foods, 10(8), 1960. https://doi.org/10.3390/foods10081960