Impact of Plant-Based Meat Alternatives on the Gut Microbiota of Consumers: A Real-World Study
Abstract
:1. Introduction
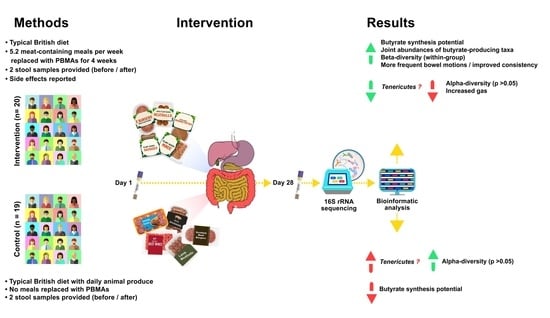
2. Materials and Methods
2.1. Participants
2.1.1. Recruitment Procedure
- No underlying health conditions requiring prescription medication.
- Aged between 18 and 55.
- A BMI between 18.5 and 29.9.
- No antibiotics in the past 6 months.
- No probiotic supplements in the past month.
- Eats red meat/poultry/fish/eggs/cheese daily.
- Does not eat plant-based meat substitutes.
- Immediate DNA family (mother, father, brother, sister) with a medical diagnosis of ulcerative colitis, Crohn’s disease, irritable bowel syndrome, or bowel cancer.
- Allergic to soya.
- History of mental health disorders or brain cancer.
- Diagnosed with a condition for which they receive NHS support.
- Positive COVID-19 diagnosis or suspected COVID-19 symptoms in the previous 6 months.
2.1.2. Randomisation and Group-Allocation Procedure
2.1.3. Plant-Based Products Consumed by the Intervention Group
2.1.4. Study Design and Procedure
2.1.5. Participant Data Collection
2.2. Gut Microbiome Analysis
2.2.1. Sample Collection
2.2.2. Extended Statistical Analysis of the Microbiome Data
- Detection of the taxa for which the between-group differences before the intervention were lower than between-group differences after the intervention (using ALDEx2 algorithm [57]);
- Calculation of the matrix of bacterial changes (abundance_after/abundance_before) using only bacteria detected by ALDEx2;
- DBA on matrix of bacteria changes using interventional group as a factor (sbp.fromADBA) to obtain ilr coordinates;
- Calculation of the balance values before and after the intervention using DBA-defined ilr coordinates;
- ANCOVA analysis for each balance using the following formula:(balance after intervention) ~ intercept + (balance before intervention) + group
- Estimation of the p-values for group factor and FDR correction.
2.2.3. Gut Microbiome Metabolic Potential Estimation
3. Results
3.1. Participant Data: Meal Consumption and Side Effects
3.2. Baseline Taxonomic Composition of the Participants’ Microbiota
3.3. Microbiome Composition Changes Due to the Intervention
3.3.1. Beta-Diversity
3.3.2. Between-Group Differential Abundance Analysis
3.3.3. Within-Group Differential Abundance Analysis
3.3.4. Alpha-Diversity Changes
3.4. Potential Changes to Butyrate-Producing Taxa
3.5. Availability of Data for Educational and Research Purposes
4. Discussion
4.1. Review of Findings
4.2. An Interesting Paradox
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, W.; Henneberg, M. Meat consumption providing a surplus energy in modern diet contributes to obesity prevalence: An ecological analysis. BMC Nutr. 2016, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Montonen, J.; Boeing, H.; Fritsche, A.; Schleicher, E.; Joost, H.-G.; Schulze, M.B.; Steffen, A.; Pischon, T. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur. J. Nutr. 2013, 52, 337–345. [Google Scholar] [CrossRef] [Green Version]
- InterAct Consortium. Association between dietary meat consumption and incident type 2 diabetes: The EPIC-InterAct study. Diabetologia 2013, 56, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Feskens, E.J.M.; Sluik, D.; van Woudenbergh, G.J. Meat Consumption, Diabetes, and Its Complications. Curr. Diabetes Rep. 2013, 13, 298–306. [Google Scholar] [CrossRef]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and Processed Meat Consumption and Risk of Incident Coronary Heart Disease, Stroke, and Diabetes Mellitus. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [Green Version]
- Bechthold, A.; Boeing, H.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Schlesinger, S.; et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1071–1090. [Google Scholar] [CrossRef] [Green Version]
- Parkin, D.M.; Boyd, L.; Walker, L.C. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br. J. Cancer 2011, 105 (Suppl. 2), S77–S81. [Google Scholar] [CrossRef] [Green Version]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [Green Version]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional Status and the Influence of the Vegan Diet on the Gut Microbiota and Human Health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef] [Green Version]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukid, F.; Rosell, C.M.; Rosene, S.; Bover-Cid, S.; Castellari, M. Non-animal proteins as cutting-edge ingredients to reformulate animal-free foodstuffs: Present status and future perspectives. Crit. Rev. food Sci. Nutr. 2021, 1–31. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Dietary Change Scenarios and Implications for Environmental, Nutrition, Human Health and Economic Dimensions of Food Sustainability. Nutrients 2019, 11, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtain, F.; Grafenauer, S. Plant-Based Meat Substitutes in the Flexitarian Age: An Audit of Products on Supermarket Shelves. Nutrients 2019, 11, 2603. [Google Scholar] [CrossRef] [Green Version]
- Michel, F.; Hartmann, C.; Siegrist, M. Consumers’ associations, perceptions and acceptance of meat and plant-based meat alternatives. Food Qual. Prefer. 2021, 87, 104063. [Google Scholar] [CrossRef]
- Gallagher, C.T.; Hanley, P.; Lane, K.E. Pattern analysis of vegan eating reveals healthy and unhealthy patterns within the vegan diet. Public Health Nutr. 2021, 1–33. [Google Scholar] [CrossRef]
- Saswattecha, K.; Kroeze, C.; Jawjit, W.; Hein, L. Assessing the environmental impact of palm oil produced in Thailand. J. Clean. Prod. 2015, 100, 150–169. [Google Scholar] [CrossRef]
- Wahyono, Y.; Hadiyanto, H.; Budihardjo, M.A.; Adiansyah, J.S. Assessing the Environmental Performance of Palm Oil Biodiesel Production in Indonesia: A Life Cycle Assessment Approach. Energies 2020, 13, 3248. [Google Scholar] [CrossRef]
- Boccia, F.; Punzo, G. A choice experiment on consumer perceptions of three generations of genetically modified foods. Appetite 2021, 161, 105158. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Clune, S.; Crossin, E.; Verghese, K. Systematic review of greenhouse gas emissions for different fresh food categories. J. Clean. Prod. 2017, 140, 766–783. [Google Scholar] [CrossRef] [Green Version]
- Soret, S.; Mejia, A.; Batech, M.; Jaceldo-Siegl, K.; Harwatt, H.; Sabaté, J. Climate change mitigation and health effects of varied dietary patterns in real-life settings throughout North America. Am. J. Clin. Nutr. 2014, 100, 490S–495S. [Google Scholar] [CrossRef] [Green Version]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef]
- Gehring, J.; Touvier, M.; Baudry, J.; Julia, C.; Buscail, C.; Srour, B.; Hercberg, S.; Péneau, S.; Kesse-Guyot, E.; Allès, B. Consumption of Ultra-Processed Foods by Pesco-Vegetarians, Vegetarians, and Vegans: Associations with Duration and Age at Diet Initiation. J. Nutr. 2021, 151, 120–131. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Thavamani, A.; Sferra, T.J.; Sankararaman, S. Meet the Meat Alternatives: The Value of Alternative Protein Sources. Curr. Nutr. Rep. 2020, 9, 346–355. [Google Scholar] [CrossRef]
- Dahl, W.J.; Rivero Mendoza, D.; Lambert, J.M. Diet, nutrients and the microbiome. Prog. Mol. Biol. Transl. Sci. 2020, 171, 237–263. [Google Scholar] [CrossRef]
- Martínez Leo, E.E.; Segura Campos, M.R. Effect of ultra-processed diet on gut microbiota and thus its role in neurodegenerative diseases. Nutrition 2020, 71, 110609. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamm, M.A. Processed food affects the gut microbiota: The revolution has started. J. Gastroenterol. Hepatol. 2020, 35, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Create a Blocked Randomisation List. Available online: https://www.sealedenvelope.com/simple-randomiser/v1/lists (accessed on 20 November 2020).
- Farm, M. Meatless Farm-Make It Meatless! Available online: https://meatlessfarm.com/ (accessed on 20 November 2020).
- Corp, I. IBM SPSS Statistics for Windows, Version 25.0; IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- Biomed, A. The Atlas Biomed Microbiome Test. Available online: https://atlasbiomed.com/uk/microbiome (accessed on 22 March 2021).
- Panek, M.; Čipčić Paljetak, H.; Barešić, A.; Perić, M.; Matijašić, M.; Lojkić, I.; Vranešić Bender, D.; Krznarić, Ž.; Verbanac, D. Methodology challenges in studying human gut microbiota-effects of collection, storage, DNA extraction and next generation sequencing technologies. Sci. Rep. 2018, 8, 5143. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Yun, K.E.; Chu, J.M.; Lee, J.Y.; Hong, C.P.; Nam, Y.D.; Jeong, J.; Han, K.; Ahn, Y.J. Performance comparison of fecal preservative and stock solutions for gut microbiome storage at room temperature. J. Microbiol. 2020, 58, 703–710. [Google Scholar] [CrossRef]
- Biomed, A. About Atlas. Available online: https://atlasbiomed.com/uk/about (accessed on 22 March 2021).
- ISO. ISO 13485:2016(en), Medical Devices—Quality Management Systems. Requirements for Regulatory Purposes; International Organization for Standards: Geneva, Switzerland, 2016. [Google Scholar]
- Chen, C.C.; Wu, W.K.; Chang, C.M.; Panyod, S.; Lu, T.P.; Liou, J.M.; Fang, Y.J.; Chuang, E.Y.; Wu, M.S. Comparison of DNA stabilizers and storage conditions on preserving fecal microbiota profiles. J. Formos. Med. Assoc. 2020, 119, 1791–1798. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Ravi, R.K.; Walton, K.; Khosroheidari, M. MiSeq: A Next Generation Sequencing Platform for Genomic Analysis. Methods Mol. Biol. 2018, 1706, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [Green Version]
- Wagner, B.D.; Grunwald, G.K.; Zerbe, G.O.; Mikulich-Gilbertson, S.K.; Robertson, C.E.; Zemanick, E.T.; Harris, J.K. On the Use of Diversity Measures in Longitudinal Sequencing Studies of Microbial Communities. Front. Microbiol. 2018, 9, 1037. [Google Scholar] [CrossRef] [Green Version]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef] [Green Version]
- Maziarz, M.; Pfeiffer, R.M.; Wan, Y.; Gail, M.H. Using standard microbiome reference groups to simplify beta-diversity analyses and facilitate independent validation. Bioinformatics 2018, 34, 3249–3257. [Google Scholar] [CrossRef] [Green Version]
- Modin, O.; Liébana, R.; Saheb-Alam, S.; Wilén, B.-M.; Suarez, C.; Hermansson, M.; Persson, F. Hill-based dissimilarity indices and null models for analysis of microbial community assembly. Microbiome 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Palarea-Albaladejo, J.; Martín-Fernández, J.A. zCompositions—R package for multivariate imputation of left-censored data under a compositional approach. Chemom. Intell. Lab. Syst. 2015, 143, 85–96. [Google Scholar] [CrossRef]
- Quinn, T.P.; Erb, I. Interpretable Log Contrasts for the Classification of Health Biomarkers: A New Approach to Balance Selection. mSystems 2020, 5, e00230-19. [Google Scholar] [CrossRef] [Green Version]
- Haynes, W. Benjamini–Hochberg Method. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; p. 78. [Google Scholar]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gloor, G. ALDEx2: ANOVA-Like Differential Expression tool for compositional data. ALDEX Man. Modul. 2015, 20, 1–11. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Efimova, D.; Tyakht, A.; Popenko, A.; Vasilyev, A.; Altukhov, I.; Dovidchenko, N.; Odintsova, V.; Klimenko, N.; Loshkarev, R.; Pashkova, M.; et al. Knomics-Biota—A system for exploratory analysis of human gut microbiota data. BioData Min. 2018, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Gonlachanvit, S.; Coleski, R.; Owyang, C.; Hasler, W. Inhibitory actions of a high fibre diet on intestinal gas transit in healthy volunteers. Gut 2004, 53, 1577–1582. [Google Scholar] [CrossRef] [Green Version]
- Klimenko, N.S.; Tyakht, A.V.; Popenko, A.S.; Vasiliev, A.S.; Altukhov, I.A.; Ischenko, D.S.; Shashkova, T.I.; Efimova, D.A.; Nikogosov, D.A.; Osipenko, D.A.; et al. Microbiome Responses to an Uncontrolled Short-Term Diet Intervention in the Frame of the Citizen Science Project. Nutrients 2018, 10, 576. [Google Scholar] [CrossRef] [Green Version]
- StataCorp. Stata|New in Stata. Available online: https://www.stata.com/new-in-stata/ (accessed on 10 May 2021).
- Lin, H.; Peddada, S.D. Analysis of microbial compositions: A review of normalization and differential abundance analysis. NPJ Biofilms Microbiomes 2020, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, Z.D.; Müller, C.L.; Miraldi, E.R.; Littman, D.R.; Blaser, M.J.; Bonneau, R.A. Sparse and Compositionally Robust Inference of Microbial Ecological Networks. PLoS Comput. Biol. 2015, 11, e1004226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egozcue, J.J.; Pawlowsky-Glahn, V. Groups of Parts and Their Balances in Compositional Data Analysis. Math. Geol. 2005, 37, 795–828. [Google Scholar] [CrossRef] [Green Version]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [Green Version]
- Rackerby, B.; Kim, H.J.; Dallas, D.C.; Park, S.H. Understanding the effects of dietary components on the gut microbiome and human health. Food Sci. Biotechnol. 2020, 29, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Shively, C.A.; Register, T.C.; Craft, S.; Yadav, H. Gut microbiome-Mediterranean diet interactions in improving host health. F1000Research 2019, 8, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patman, G. Lactobacillus acidophilus opens the door to butyrate. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Rothschild, D.; Leviatan, S.; Hanemann, A.; Cohen, Y.; Weissbrod, O.; Segal, E. An atlas of robust microbiome associations with phenotypic traits based on large-scale cohorts from two continents. bioRxiv 2020. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef] [Green Version]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Velázquez, O.C.; Lederer, H.M.; Rombeau, J.L. Butyrate and the colonocyte. Dig. Dis. Sci. 1996, 41, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in Energy Metabolism: There Is Still More to Learn. Trends Endocrinol. Metab. 2021, 32, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef] [Green Version]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. mSystems 2017, 2, e00130-17. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Chávez, F.; Zhang, L.F.; Faber, F.; Lopez, C.A.; Byndloss, M.X.; Olsan, E.E.; Xu, G.; Velazquez, E.M.; Lebrilla, C.B.; Winter, S.E.; et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe 2016, 19, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavel, F.M.; Vesa, C.M.; Gheorghe, G.; Diaconu, C.C.; Stoicescu, M.; Munteanu, M.A.; Babes, E.E.; Tit, D.M.; Toma, M.M.; Bungau, S. Highlighting the Relevance of Gut Microbiota Manipulation in Inflammatory Bowel Disease. Diagnostics 2021, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Prosberg, M.; Bendtsen, F.; Vind, I.; Petersen, A.M.; Gluud, L.L. The association between the gut microbiota and the inflammatory bowel disease activity: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2016, 51, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. MBio 2014, 5, e00889. [Google Scholar] [CrossRef] [Green Version]
- Haro, C.; Montes-Borrego, M.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Tinahones, F.J.; Landa, B.B.; et al. Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population. J. Clin. Endocrinol. Metab. 2016, 101, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Rosés, C.; Cuevas-Sierra, A.; Quintana, S.; Riezu-Boj, J.I.; Martínez, J.A.; Milagro, F.I.; Barceló, A. Gut Microbiota Bacterial Species Associated with Mediterranean Diet-Related Food Groups in a Northern Spanish Population. Nutrients 2021, 13, 636. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef] [Green Version]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef]
- De Filippis, F.; Pasolli, E.; Ercolini, D. Newly Explored Faecalibacterium Diversity Is Connected to Age, Lifestyle, Geography, and Disease. Curr. Biol. 2020, 30, 4932–4943.e4. [Google Scholar] [CrossRef]
- Shevlyakov, A.; Nikogosov, D.; Stewart, L.-A.; Toribio-Mateas, M. Reference values for intake of 6 types of soluble and insoluble fibre in healthy UK inhabitants based on the UK Biobank data. Public Health Nutr. 2021, 1–41. [Google Scholar] [CrossRef]
- Molan, A.L.; Liu, Z.; Plimmer, G. Evaluation of the effect of blackcurrant products on gut microbiota and on markers of risk for colon cancer in humans. Phytother. Res. 2014, 28, 416–422. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- Song, Y.; Wu, M.-S.; Tao, G.; Lu, M.-W.; Lin, J.; Huang, J.-Q. Feruloylated oligosaccharides and ferulic acid alter gut microbiome to alleviate diabetic syndrome. Food Res. Int. 2020, 137, 109410. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of lignans in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microb. Cell Factories 2020, 19, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef]
- Patrone, V.; Minuti, A.; Lizier, M.; Miragoli, F.; Lucchini, F.; Trevisi, E.; Rossi, F.; Callegari, M.L. Differential effects of coconut versus soy oil on gut microbiota composition and predicted metabolic function in adult mice. BMC Genomics 2018, 19, 808. [Google Scholar] [CrossRef]
- Chen, P.Y.; Li, S.; Koh, Y.C.; Wu, J.C.; Yang, M.J.; Ho, C.T.; Pan, M.H. Oolong Tea Extract and Citrus Peel Polymethoxyflavones Reduce Transformation of l-Carnitine to Trimethylamine-N-Oxide and Decrease Vascular Inflammation in l-Carnitine Feeding Mice. J. Agric. Food Chem. 2019, 67, 7869–7879. [Google Scholar] [CrossRef]
- Wu, W.-K.; Panyod, S.; Liu, P.-Y.; Chen, C.-C.; Kao, H.-L.; Chuang, H.-L.; Chen, Y.-H.; Zou, H.-B.; Kuo, H.-C.; Kuo, C.-H.; et al. Characterization of TMAO productivity from carnitine challenge facilitates personalized nutrition and microbiome signatures discovery. Microbiome 2020, 8, 162. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Tan, X.Y.; Li, Q.J.; Liao, G.C.; Fang, A.P.; Zhang, D.M.; Chen, P.Y.; Wang, X.Y.; Luo, Y.; Long, J.A.; et al. Trimethylamine N-oxide, a gut microbiota-dependent metabolite of choline, is positively associated with the risk of primary liver cancer: A case-control study. Nutr. Metab. 2018, 15, 81. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.V.A.; Burnet, P.W.J. Microbiome: Should we diversify from diversity? Gut Microbes 2016, 7, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Ackoff, R.L.; Gharajedaghi, J. Reflections on systems and their models. Syst. Res. 1996, 13, 13–23. [Google Scholar] [CrossRef]
- Sturmberg, J.P. Health and Disease Are Dynamic Complex-Adaptive States Implications for Practice and Research. Front. Psychiatry 2021, 12, 354. [Google Scholar] [CrossRef]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.e5. [Google Scholar] [CrossRef]
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Slavatore, M.; Fornaro, M.; Bhugra, D. Mediterranean Diet and its Benefits on Health and Mental Health: A Literature Review. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Sánchez, M.L.; García-Vigara, A.; Hidalgo-Mora, J.J.; García-Pérez, M.; Tarín, J.; Cano, A. Mediterranean diet and health: A systematic review of epidemiological studies and intervention trials. Maturitas 2020, 136, 25–37. [Google Scholar] [CrossRef]
- Mancini, J.G.; Filion, K.B.; Atallah, R.; Eisenberg, M.J. Systematic Review of the Mediterranean Diet for Long-Term Weight Loss. Am. J. Med. 2016, 129, 407–415.e4. [Google Scholar] [CrossRef] [Green Version]
- Guasch-Ferré, M.; Salas-Salvadó, J.; Ros, E.; Estruch, R.; Corella, D.; Fitó, M.; Martínez-González, M.A. The PREDIMED trial, Mediterranean diet and health outcomes: How strong is the evidence? Nutr. Metab. Cardiovasc. Dis. 2017, 27, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Beardsworth, A.; Bryman, A. Meat consumption and meat avoidance among young people. Br. Food J. 2004, 106, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Hobbs-Grimmer, D.A.; Givens, D.I.; Lovegrove, J.A. Associations between red meat, processed red meat and total red and processed red meat consumption, nutritional adequacy and markers of health and cardio-metabolic diseases in British adults: A cross-sectional analysis using data from UK National Diet and Nutrition Survey. Eur. J. Nutr. 2021, 60, 2979–2997. [Google Scholar] [CrossRef]
- Stoll-Kleemann, S.; Schmidt, U.J. Reducing meat consumption in developed and transition countries to counter climate change and biodiversity loss: A review of influence factors. Reg. Environ. Chang. 2017, 17, 1261–1277. [Google Scholar] [CrossRef] [Green Version]
- Boukid, F. Plant-based meat analogues: From niche to mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- Public Health England. Salt Targets 2017: Second Progress Report; Public Health England: London, UK, 2017.
- Public Health England. Salt Reduction Targets for 2024; Public Health England: London, UK, 2020.
- Gebhardt, B. Plant-Based for the Future. Insights on European Consumer and Expert Opinions; EIT Food, University of Hohenheim, Department of Agricultural Market: Stuttgart, Germany, 2021. [Google Scholar] [CrossRef]
- Gillespie, S. Epidemics and food systems: What gets framed, gets done. Food Secur. 2020, 12, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Noor, S.; Tippairote, T.; Dadar, M.; Menzel, A.; Bjørklund, G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin. Immunol. 2020, 215, 108409. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Iaccarino, G.; Czarlewski, W.; Haahtela, T.; Anto, A.; Akdis, C.A.; Blain, H.; Canonica, G.W.; Cardona, V.; et al. Is diet partly responsible for differences in COVID-19 death rates between and within countries? Clin. Transl. Allergy 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson Bellamy, A.; Furness, E.; Nicol, P.; Pitt, H.; Taherzadeh, A. Shaping more resilient and just food systems: Lessons from the COVID-19 Pandemic. Ambio 2021, 50, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Keenan, J.M. COVID, resilience, and the built environment. Environ. Syst. Decis. 2020, 40, 216–221. [Google Scholar] [CrossRef]
- Tavakol, Z.; Ghannadi, S.; Tabesh, M.R.; Halabchi, F.; Noormohammadpour, P.; Akbarpour, S.; Alizadeh, Z.; Nezhad, M.H.; Reyhan, S.K. Relationship between physical activity, healthy lifestyle and COVID-19 disease severity; a cross-sectional study. J. Public Health 2021. [Google Scholar] [CrossRef]
- Wang, J.; Yeoh, E.K.; Yung, T.K.C.; Wong, M.C.S.; Dong, D.; Chen, X.; Chan, M.K.Y.; Wong, E.L.Y.; Wu, Y.; Guo, Z.; et al. Change in eating habits and physical activities before and during the COVID-19 pandemic in Hong Kong: A cross-sectional study via random telephone survey. J. Int. Soc. Sports Nutr. 2021, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Abouzid, M.; El-Sherif, D.M.; Eltewacy, N.K.; Dahman, N.B.H.; Okasha, S.A.; Ghozy, S.; Islam, S.M.S.; Elburki, A.R.F.; Ali, A.A.M.; Hasan, M.A.; et al. Influence of COVID-19 on lifestyle behaviors in the Middle East and North Africa Region: A survey of 5896 individuals. J. Transl. Med. 2021, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Kolokotroni, O.; Mosquera, M.C.; Quattrocchi, A.; Heraclides, A.; Demetriou, C.; Philippou, E. Lifestyle habits of adults during the COVID-19 pandemic lockdown in Cyprus: Evidence from a cross-sectional study. BMC Public Health 2021, 21, 786. [Google Scholar] [CrossRef]
- Abdulah, D.M.; Hassan, A.B. Relation of Dietary Factors with Infection and Mortality Rates of COVID-19 across the World. J. Nutr. Health Aging 2020, 24, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Ogueji, I.A.; Okoloba, M.M.; Demoko Ceccaldi, B.M. Coping strategies of individuals in the United Kingdom during the COVID-19 pandemic. Curr. Psychol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Verain, M.; Dagevos, H.; Antonides, G. Flexitarianism: A range of sustainable food styles. In Handbook of Research on Sustainable Consumption; Reisch, L., Thogersen, J., Eds.; Edward Elgar Publishing: Cheltenham, UK, 2015; pp. 209–223. [Google Scholar]
- Derbyshire, E.J. Flexitarian Diets and Health: A Review of the Evidence-Based Literature. Front. Nutr. 2016, 3, 55. [Google Scholar] [CrossRef] [Green Version]
- Kemper, J.A.; White, S.K. Young adults’ experiences with flexitarianism: The 4Cs. Appetite 2021, 160, 105073. [Google Scholar] [CrossRef]
- Plante, C.N.; Rosenfeld, D.L.; Plante, M.; Reysen, S. The role of social identity motivation in dietary attitudes and behaviors among vegetarians. Appetite 2019, 141, 104307. [Google Scholar] [CrossRef]
- Dakin, B.C.; Ching, A.E.; Teperman, E.; Klebl, C.; Moshel, M.; Bastian, B. Prescribing vegetarian or flexitarian diets leads to sustained reduction in meat intake. Appetite 2021, 164, 105285. [Google Scholar] [CrossRef] [PubMed]
- Mintel. Meat-Free Foods-UK-May 2017-Market Research Report; Mintel: London, UK, 2017. [Google Scholar]
- Bianchi, F.; Dorsel, C.; Garnett, E.; Aveyard, P.; Jebb, S.A. Interventions targeting conscious determinants of human behaviour to reduce the demand for meat: A systematic review with qualitative comparative analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 102. [Google Scholar] [CrossRef] [Green Version]
- Higgs, S. Social norms and their influence on eating behaviours. Appetite 2015, 86, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chaudhary, A.; Mathys, A. Nutritional and environmental losses embedded in global food waste. Resour. Conserv. Recycl. 2020, 160, 104912. [Google Scholar] [CrossRef]
- Dagevos, H. Finding flexitarians: Current studies on meat eaters and meat reducers. Trends Food Sci. Technol. 2021, 114, 530–539. [Google Scholar] [CrossRef]
- Michel, F.; Sanchez-Siles, L.M.; Siegrist, M. Predicting how consumers perceive the naturalness of snacks: The usefulness of a simple index. Food Qual. Prefer. 2021, 94, 104295. [Google Scholar] [CrossRef]
- Phan, U.T.; Chambers, E.T. Motivations for choosing various food groups based on individual foods. Appetite 2016, 105, 204–211. [Google Scholar] [CrossRef]
- Hess, J.M.; Jonnalagadda, S.S.; Slavin, J.L. What Is a Snack, Why Do We Snack, and How Can We Choose Better Snacks? A Review of the Definitions of Snacking, Motivations to Snack, Contributions to Dietary Intake, and Recommendations for Improvement. Adv. Nutr. 2016, 7, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Yates, L.; Warde, A. The evolving content of meals in Great Britain. Results of a survey in 2012 in comparison with the 1950s. Appetite 2015, 84, 299–308. [Google Scholar] [CrossRef]
- Chandler, P.D.; Balasubramanian, R.; Paynter, N.; Giulianini, F.; Fung, T.; Tinker, L.F.; Snetselaar, L.; Liu, S.; Eaton, C.; Tobias, D.K.; et al. Metabolic signatures associated with Western and Prudent dietary patterns in women. Am. J. Clin. Nutr. 2020, 112, 268–283. [Google Scholar] [CrossRef]
- Clatici, V.G.; Voicu, C.; Voaides, C.; Roseanu, A.; Icriverzi, M.; Jurcoane, S. Diseases of Civilization—Cancer, Diabetes, Obesity and Acne—the Implication of Milk, IGF-1 and mTORC1. Maedica 2018, 13, 273–281. [Google Scholar] [CrossRef]
- Global Plant Based Meat Market—Analysis by Source, by Product, by Region, by Country (2020 Edition): Market Insights, COVID-19 Impact, Competition and Forecast (2020–2025); Azoth Analytics: Dublin, Ireland, 2021.
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F. Animal and Plant Protein Sources and Cardiometabolic Health. Adv. Nutr. 2019, 10, S351–S366. [Google Scholar] [CrossRef] [PubMed]
- Hemler, E.C.; Hu, F.B. Plant-Based Diets for Cardiovascular Disease Prevention: All Plant Foods Are Not Created Equal. Curr. Atheroscler. Rep. 2019, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [Green Version]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- Lane, M.; Howland, G.; West, M.; Hockey, M.; Marx, W.; Loughman, A.; O’Hely, M.; Jacka, F.; Rocks, T. The effect of ultra-processed very low-energy diets on gut microbiota and metabolic outcomes in individuals with obesity: A systematic literature review. Obes. Res. Clin. Pract. 2020, 14, 197–204. [Google Scholar] [CrossRef]
- Sandall, A.M.; Cox, S.R.; Lindsay, J.O.; Gewirtz, A.T.; Chassaing, B.; Rossi, M.; Whelan, K. Emulsifiers Impact Colonic Length in Mice and Emulsifier Restriction is Feasible in People with Crohn’s Disease. Nutrients 2020, 12, 2827. [Google Scholar] [CrossRef]
- Miranda, J.; Portocarrero, A.; Freire, A.; Abuin, C.; Saez, A. Advantages, Disadvantages, and Future Trends on the Use of Design of Experiments in Cross-Over Trials in Nutritional Clinical Investigation; IGI Global: Hershey, PA, USA, 2020; pp. 158–173. [Google Scholar]
- Mills, E.J.; Chan, A.-W.; Wu, P.; Vail, A.; Guyatt, G.H.; Altman, D.G. Design, analysis, and presentation of crossover trials. Trials 2009, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Veganuary. Veganuary—The International Movement Inspiring People to Try Vegan! Available online: https://veganuary.com/ (accessed on 20 April 2021).
- Evans, C.E.L. Dietary fibre and cardiovascular health: A review of current evidence and policy. Proc. Nutr. Soc. 2020, 79, 61–67. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwinowicz, K.; Choroszy, M.; Waszczuk, E. Changes in the composition of the human intestinal microbiome in alcohol use disorder: A systematic review. Am. J. Drug Alcohol. Abus. 2020, 46, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Alvarez, L.; Xu, H.; Martinez-Tellez, B. Influence of Exercise on the Human Gut Microbiota of Healthy Adults: A Systematic Review. Clin. Transl. Gastroenterol. 2020, 11, e00126. [Google Scholar] [CrossRef] [PubMed]







| Per 100 g | Burger | Sausage | Mince | Sausage Patty | Meatballs | Mean ± SD |
|---|---|---|---|---|---|---|
| Calories (kcal) | 230 | 234 | 199 | 233 | 223 | 227.95 ± 7.07 |
| Protein (grams) | 17.1 | 14.4 | 19.1 | 16.6 | 15 | 15.23 ± 1.20 |
| Fibre (grams) | 3.7 | 3.2 | 4.9 | 2.5 | 4.2 | 3.64 ± 0.82 |
| Fat (grams) | 14.8 | 15.9 | 10.9 | 15 | 11.9 | 11.69 ± 5.16 |
| (of which saturates) | 4.7 | 5 | 3.9 | 4.2 | 0.8 | 2.00 ± 2.97 |
| Carbohydrate (grams) | 5.3 | 6.9 | 7.8 | 10 | 11.8 | 6.70 ± 0.28 |
| (of which sugars) | 0.3 | 0.3 | 0.1 | 0 | 0.9 | 0.30 ± 0.00 |
| Salt (grams) | 1.49 | 1.27 | 0.62 | 1.26 | 1.36 | 1.38 ± 0.16 |
| Cholesterol (grams) | 0 | 0 | 0 | 0 | 0 | |
| Protein source | Pea and rice | Pea and rice | Soy, pea and rice | Pea | Pea |
| Protein Source | Daidzein (mg/kg) | Secoisolariciresonol (mg/kg) | Ferulic Acid (mg/kg) | Vitamin K1 (μg/100 g) | Vitamin K2(MK4) (μg/100 g) | Vitamin K2(MK7)(μg/100 g) | Genistein (mg/kg) | Lutein (mg/kg) | Zeaxanthin (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Pea Flour | <0.5 | <0.5 | 3.5 | 11.8 | 0.35 | 0.17 | 0.75 | 1.73 | <0.5 |
| Pea Protein Concentrate (Dry Fractionated) | <0.5 | <0.5 | 2.5 | 12 | 1.92 | 2.29 | 0.85 | 12.29 | <0.5 |
| Dehulled Peas | <0.5 | <0.5 | 2.9 | 13.6 | 0.46 | 0.16 | 0.15 | 7.92 | <0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toribio-Mateas, M.A.; Bester, A.; Klimenko, N. Impact of Plant-Based Meat Alternatives on the Gut Microbiota of Consumers: A Real-World Study. Foods 2021, 10, 2040. https://doi.org/10.3390/foods10092040
Toribio-Mateas MA, Bester A, Klimenko N. Impact of Plant-Based Meat Alternatives on the Gut Microbiota of Consumers: A Real-World Study. Foods. 2021; 10(9):2040. https://doi.org/10.3390/foods10092040
Chicago/Turabian StyleToribio-Mateas, Miguel A., Adri Bester, and Natalia Klimenko. 2021. "Impact of Plant-Based Meat Alternatives on the Gut Microbiota of Consumers: A Real-World Study" Foods 10, no. 9: 2040. https://doi.org/10.3390/foods10092040
APA StyleToribio-Mateas, M. A., Bester, A., & Klimenko, N. (2021). Impact of Plant-Based Meat Alternatives on the Gut Microbiota of Consumers: A Real-World Study. Foods, 10(9), 2040. https://doi.org/10.3390/foods10092040