Brown Algae as Functional Food Source of Fucoxanthin: A Review
Abstract
:1. Introduction
2. Function and Availability of Fucoxanthin in Seaweed
3. Biosynthesis of Fucoxanthin in Seaweed
4. Metabolism, Bioavailability and Bioaccessibility of Fucoxanthin
5. Potential Biological Activities of Fucoxanthin
5.1. Antioxidant Activity
5.2. Anti-Inflammatory Activity
5.3. Anti-Obesity Activity
5.4. Antidiabetic Activity
5.5. Anticancer Activity
5.6. Other Activities
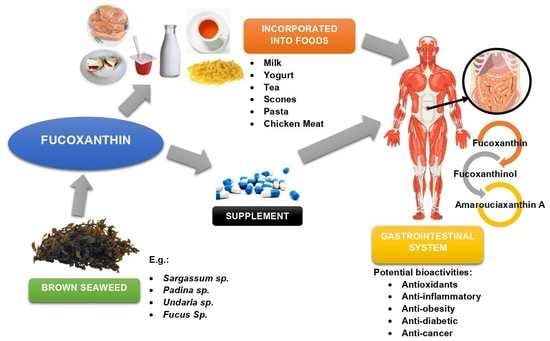
6. Fucoxanthin as Functional Food Ingredients
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barsanti, L.; Coltelli, P.; Evangelista, V.; Frassanito, A.M.; Passarelli, V.; Vesentini, N.; Gualtieri, P. Oddities and Curiosities in the Algal World. In Algal Toxins: Nature, Occurrence, Effect and Detection; Evangelista, V., Barsanti, L., Frassanito, A.M., Passarelli, V., Gualtieri, P., Eds.; Springer Science & Business Media: Pisa, Italy, 2008; pp. 353–356. [Google Scholar]
- Qin, S.; Lin, H.; Jiang, P. Advances in genetic engineering of marine algae. Biotechnol. Adv. 2012, 30, 1602–1613. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration. What Is Seaweed? Available online: https://oceanservice.noaa.gov/facts/seaweed.html (accessed on 25 August 2021).
- Zainee, N.F.A.; Rozaimi, M. Influence of monsoonal storm disturbance on the diversity of intertidal macroalgae along the eastern coast of Johor (Malaysia). Reg. Stud. Mar. Sci. 2020, 40, 101481. [Google Scholar] [CrossRef]
- Terasaki, M.; Kawagoe, C.; Ito, A.; Kumon, H.; Narayan, B.; Hosokawa, M.; Miyashita, K. Spatial and seasonal variations in the biofunctional lipid substances (fucoxanthin and fucosterol) of the laboratory-grown edible Japanese seaweed (Sargassum horneri Turner) cultured in the open sea. Saudi J. Biol. Sci. 2017, 24, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Airanthi, M.W.A.; Hosokawa, M.; Miyashita, K. Comparative antioxidant activity of edible Japanese brown seaweeds. J. Food Sci. 2011, 76, C104–C111. [Google Scholar] [CrossRef]
- Brownlee, I.; Fairclough, A.; Hall, A.; Paxman, J. Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011. [Google Scholar]
- Hillier, K.; Rakkar, M. Alginic acid. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–3. [Google Scholar]
- Kumar, C.S.; Ganesan, P.; Suresh, P.; Bhaskar, N. Seaweeds as a source of nutritionally beneficial compounds—A review. J. Food Sci. Technol. 2008, 45, 1–13. [Google Scholar]
- Asmida, I.; Akmal, N.; Ahmad, I.; Sarah Diyana, M. Biodiversity of macroalgae in Blue Lagoon, the Straits of Malacca, Malaysia and some aspects of changes in species composition. Sains Malays. 2017, 46, 1–7. [Google Scholar]
- Badar, S.N.; Mohammad, M.; Emdadi, Z.; Yaakob, Z. Algae and their growth requirements for bioenergy: A review. Biofuels. 2021, 12, 307–325. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M. Current status of the algae production industry in Europe: An emerging sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Cai, J. Global Status of Seaweed Production, Trade and Utilization; Food and Agriculture Organisation of United Nations: Rome, Italy, 2021; pp. 1–18. [Google Scholar]
- Shen, Y.; Li, J.; Gu, R.; Yue, L.; Wang, H.; Zhan, X.; Xing, B. Carotenoid and superoxide dismutase are the most effective antioxidants participating in ROS scavenging in phenanthrene accumulated wheat leaf. Chemosphere 2018, 197, 513–525. [Google Scholar] [CrossRef]
- Englert, G.; Bjørnland, T.; Liaaen-Jensen, S. 1D and 2D NMR study of some allenic carotenoids of the fucoxanthin series. Magn. Reson. Chem. 1990, 28, 519–528. [Google Scholar] [CrossRef]
- Sangeetha, R.K.; Bhaskar, N.; Divakar, S.; Baskaran, V. Bioavailability and metabolism of fucoxanthin in rats: Structural characterization of metabolites by LC-MS (APCI). Mol. Cell. Biochem. 2010, 333, 299–310. [Google Scholar] [CrossRef]
- Bertrand, M. Carotenoid biosynthesis in diatoms. Photosynth. Res. 2010, 106, 89–102. [Google Scholar] [CrossRef]
- Mikami, K.; Hosokawa, M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int. J. Mol. Sci. 2013, 14, 13763–13781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egeland. Carotenoids. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2016; Volume 6, pp. 507–563. [Google Scholar]
- Lohr, M. Carotenoids. In The Chlamydomonas Sourcebook, 2nd ed.; Harris, E.H., Stern, D.B., Witman, G.B., Eds.; Academic Press: Amsterdam, The Netherlands, 2009; pp. 799–817. [Google Scholar]
- Abu-Ghannam, N.; Shannon, E. Seaweed carotenoid, fucoxanthin, as functional food. Microb. Funct. Foods Nutraceuticals 2017, 1, 39–64. [Google Scholar]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-F.; Shen, Y.-C.; Venkatakrishnan, K.; Wang, C.K. Food for Eye Health: Carotenoids and Omega-3 Fatty Acids. Encycl. Food Chem. 2019, 3, 313–322. [Google Scholar]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the sea: Algae as source of bioactive carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Zheng, J.; Piao, M.J.; Keum, Y.S.; Kim, H.S.; Hyun, J.W. Fucoxanthin protects cultured human keratinocytes against oxidative stress by blocking free radicals and inhibiting apoptosis. Biomol. Ther. 2013, 21, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Jin, E.-S.; Polle, J.E.; Lee, H.-K.; Hyun, S.-M.; Chang, M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J. Microbiol. Biotechnol. 2003, 13, 165–174. [Google Scholar]
- Pyszniak, A.; Gibbs, S.P. Immunocytochemical localization of photosystem I and the fucoxanthin-chlorophylla/c light-harvesting complex in the diatom Phaeodactylum tricornutum. Protoplasma 1992, 166, 208–217. [Google Scholar] [CrossRef]
- Li, J.; Bidigare, R.R.; Laws, E.A. Effects of Macondo oil on phytoplankton from Grand Isle, Louisiana. J. Environ. Anal. Toxicol. 2017, 7, 2161-0525. [Google Scholar] [CrossRef] [Green Version]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Foo, S.C.; Khoo, K.S.; Ooi, C.W.; Show, P.L.; Khong, N.M.; Yusoff, F.M. Meeting sustainable development goals: Alternative extraction processes for fucoxanthin in algae. Front. Bioeng. Biotechnol. 2021, 8, 546067. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Barbosa, M.; Oliveira, A.P.; Azevedo, I.C.; Sousa-Pinto, I.; Valentão, P.; Andrade, P.B. The pigments of kelps (Ochrophyta) as part of the flexible response to highly variable marine environments. J. Appl. Phycol. 2016, 28, 3689–3696. [Google Scholar] [CrossRef]
- Fariman, G.A.; Shastan, S.J.; Zahedi, M.M. Seasonal variation of total lipid, fatty acids, fucoxanthin content, and antioxidant properties of two tropical brown algae (Nizamuddinia zanardinii and Cystoseira indica) from Iran. J. Appl. Phycol. 2016, 28, 1323–1331. [Google Scholar] [CrossRef]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of Recoverable Functional Lipid Components of Several Brown Seaweeds (Phaeophyta) from Japan with Special Reference to Fucoxanthin and Fucosterol Contents. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Karkhaneh Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghasempour, A. Seasonal variation of fucoxanthin content in four species of brown seaweeds from Qeshm Island, Persian Gulf and evaluation of their antibacterial and antioxidant activities. Iran. J. Fish. Sci. 2020, 19, 2394–2408. [Google Scholar]
- Zarekarizi, A.; Hoffmann, L.; Burritt, D. Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. J. Appl. Phycol. 2019, 31, 281–299. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Angove, M.; Morton, D. A screening method for cardiovascular active compounds in marine algae. J. Chromatogr. A 2018, 1550, 57–62. [Google Scholar] [CrossRef]
- Norra, I.; Aminah, A.; Suri, R.; Arif Zaidi, J. Effect of drying temperature on the content of fucoxanthin, phenolic and antioxidant activity of Malaysian brown seaweed, Sargassum sp. J. Trop. Agric. Food Sci. 2017, 45, 25–36. [Google Scholar]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Yau, S.K.; Khong, N.M.; Chan, K.W.; Ebrahimi, M. Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. J. Biotechnol. 2017, 241, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, H.; Pee, P.P.; Kee, S.H.Y.; Ow, J.T.; Yan, S.W.; Chew, L.Y.; Kong, K.W. Malaysian brown seaweeds Sargassum siliquosum and Sargassum polycystum: Low density lipoprotein (LDL) oxidation, angiotensin converting enzyme (ACE), α-amylase, and α-glucosidase inhibition activities. Food Res. Int. 2017, 99, 950–958. [Google Scholar] [CrossRef]
- Yip, W.H.; Joe, L.S.; Mustapha, W.A.W.; Maskat, M.Y.; Said, M. Characterisation and stability of pigments extracted from Sargassum binderi obtained from Semporna, Sabah. Sains Malays. 2014, 43, 1345–1354. [Google Scholar]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Taher, M.; Miyashita, K.; Ramli, N. Analysis of fucoxanthin content and purification of all-trans-fucoxanthin from Turbinaria turbinata and Sargassum plagyophyllum by SiO2 open column chromatography and reversed phase-HPLC. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1340–1354. [Google Scholar] [CrossRef]
- Savira, A.; Amin, M.; Alamsjah, M. The effect of different type of solvents on the antioxidant activity of fucoxanthin extract from brown seaweed Sargassum duplicatum. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surabaya, Indonesia, 10 September 2021; p. 012010. [Google Scholar]
- Sumandiarsa, I.K.; Bengen, D.G.; Santoso, J.; Januar, H.I. Spatial-temporal effect on proximate, trace elements, alginate, and fucoxanthin contents, of Sargassum polycystum brown seaweed. J. Hunan Univ. Nat. Sci. 2021, 48, 66–79. [Google Scholar]
- Nurrochmad, A.; Wirasti, W.; Dirman, A.; Lukitaningsih, E.; Rahmawati, A.; Fakhrudin, N. Effects of Antioxidant, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase of The Extract and Fraction From Turbinaria decurrens Bory. Indones. J. Pharm. 2018, 29, 188. [Google Scholar] [CrossRef]
- Susanto, E.; Fahmi, A.S.; Abe, M.; Hosokawa, M.; Miyashita, K. Lipids, fatty acids, and fucoxanthin content from temperate and tropical brown seaweeds. Aquat. Procedia 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Maeda, H.; Fukuda, S.; Izumi, H.; Saga, N. Anti-oxidant and fucoxanthin contents of brown alga Ishimozuku (Sphaerotrichia divaricata) from the West Coast of Aomori, Japan. Mar. Drugs 2018, 16, 255. [Google Scholar] [CrossRef] [Green Version]
- Kanda, H.; Kamo, Y.; Machmudah, S.; Goto, M. Extraction of fucoxanthin from raw macroalgae excluding drying and cell wall disruption by liquefied dimethyl ether. Mar. Drugs 2014, 12, 2383–2396. [Google Scholar] [CrossRef]
- Nomura, M.; Kamogawa, H.; Susanto, E.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseira hakodatensis (Yendo) from the northern seashore of Japan. J. Appl. Phycol. 2013, 25, 1159–1169. [Google Scholar] [CrossRef]
- Chen, C.-R.; Lin, D.-M.; Chang, C.-M.J.; Chou, H.-N.; Wu, J.-J. Supercritical carbon dioxide anti-solvent crystallization of fucoxanthin chromatographically purified from Hincksia mitchellae PC Silva. J. Supercrit. Fluids 2017, 119, 1–8. [Google Scholar] [CrossRef]
- Getachew, A.T.; Saravana, P.S.; Cho, Y.J.; Woo, H.C.; Chun, B.S. Concurrent extraction of oil from roasted coffee (Coffea arabica) and fucoxanthin from brown seaweed (Saccharina japonica) using supercritical carbon dioxide. J. CO2 Util. 2018, 25, 137–146. [Google Scholar] [CrossRef]
- Sivagnanam, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef]
- Ye, Y.; Sun, J.; Wang, L.; Zhu, J.; Cui, W.; Hou, H.; Zhang, J.; Zhou, C.; Yan, X. Isolation and purification of fucoxanthin from brown seaweed Sargassum horneri using open ODS column chromatography and ethanol precipitation. Molecules 2021, 26, 3777. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Liu, Y.; Wang, Q.; Gao, X.; Gong, Q. Effects of temperature and salinity on the growth and biochemical composition of the brown alga Sargassum fusiforme (Fucales, Phaeophyceae). J. Appl. Phycol. 2019, 31, 3061–3068. [Google Scholar] [CrossRef]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M. Ultrasound-assisted extraction of fucoxanthin from Sargassum angustifolium and Cystoseira indica brown algae. J. Food Process. Preserv. 2021, 45, e15929. [Google Scholar] [CrossRef]
- Raji, V.; Loganathan, C.; Sadhasivam, G.; Kandasamy, S.; Poomani, K.; Thayumanavan, P. Purification of fucoxanthin from Sargassum wightii Greville and understanding the inhibition of angiotensin 1-converting enzyme: An In Vitro and In Silico studies. Int. J. Biol. Macromol. 2020, 148, 696–703. [Google Scholar] [CrossRef]
- Raguraman, V.; MubarakAli, D.; Narendrakumar, G.; Thirugnanasambandam, R.; Kirubagaran, R.; Thajuddin, N. Unraveling rapid extraction of fucoxanthin from Padina tetrastromatica: Purification, characterization and biomedical application. Process Biochem. 2018, 73, 211–219. [Google Scholar] [CrossRef]
- Verma, P.; Kumar, M.; Mishra, G.; Sahoo, D. Multivariate analysis of fatty acid and biochemical constitutes of seaweeds to characterize their potential as bioresource for biofuel and fine chemicals. Bioresour. Technol. 2017, 226, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Enzymatic extraction of fucoxanthin from brown seaweeds. Int. J. Food Sci. Technol. 2018, 53, 2195–2204. [Google Scholar] [CrossRef] [Green Version]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Optimisation of fucoxanthin extraction from Irish seaweeds by response surface methodology. J. Appl. Phycol. 2017, 29, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Heffernan, N.; Smyth, T.; FitzGerald, R.J.; Vila-Soler, A.; Mendiola, J.; Ibáñez, E.; Brunton, N.P. Comparison of extraction methods for selected carotenoids from macroalgae and the assessment of their seasonal/spatial variation. Innov. Food Sci. Emerg. Technol. 2016, 37, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Nunes, N.; Leça, J.M.; Pereira, A.C.; Pereira, V.; Ferraz, S.; Barreto, M.C.; Marques, J.C.; de Carvalho, M.P. Evaluation of fucoxanthin contents in seaweed biomass by vortex-assisted solid-liquid microextraction using high-performance liquid chromatography with photodiode array detection. Algal Res. 2019, 42, 101603. [Google Scholar] [CrossRef]
- Conde, E.; Moure, A.; Domínguez, H. Supercritical CO2 extraction of fatty acids, phenolics and fucoxanthin from freeze-dried Sargassum muticum. J. Appl. Phycol. 2015, 27, 957–964. [Google Scholar] [CrossRef]
- Ryabushko, V.I.; Prazukin, A.V.; Popova, E.V.; Nekhoroshev, M.V. Fucoxanthin of the brown alga Cystoseira barbata (Stackh.) C. Agardh from the Black Sea. J. Black Sea/Mediterr. Environ. 2014, 20, 108–113. [Google Scholar]
- Billakanti, J.M.; Catchpole, O.J.; Fenton, T.A.; Mitchell, K.A.; MacKenzie, A.D. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem. 2013, 48, 1999–2008. [Google Scholar] [CrossRef]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Crowell, D.N.; Huizinga, D.H. Protein isoprenylation: The fat of the matter. Trends Plant Sci. 2009, 14, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, L.O.; Stålberg, K.G.; Hoglund, A.-S. Seed-specific overexpression of an endogenous Arabidopsis phytoene synthase gene results in delayed germination and increased levels of carotenoids, chlorophyll, and abscisic acid. Plant Physiol. 2003, 132, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Jiang, J.-G.; Wang, F. Molecular phylogenies and evolution of crt genes in algae. Crit. Rev. Biotechnol. 2007, 27, 77–91. [Google Scholar] [CrossRef]
- Li, F.; Vallabhaneni, R.; Wurtzel, E.T. PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol. 2008, 146, 1333–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandmann, G. Evolution of carotene desaturation: The complication of a simple pathway. Arch. Biochem. Biophys. 2009, 483, 169–174. [Google Scholar] [CrossRef]
- Breitenbach, J.; Kuntz, M.; Takaichi, S.; Sandmann, G. Catalytic properties of an expressed and purified higher plant typeζ-carotene desaturase from Capsicum annuum. Eur. J. Biochem. 1999, 265, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.; Ohad, I.; Beyer, P.; Hirschberg, J. Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiol. 2004, 136, 4246–4255. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.X.; Blouin, N.A.; Zhuang, Y.; Zäuner, S.; Prochnik, S.E.; Lindquist, E.; Lin, S.; Benning, C.; Lohr, M.; Yarish, C. Porphyra (Bangiophyceae) transcriptomes provide insights into red algal development and metabolism. J. Phycol. 2012, 48, 1328–1342. [Google Scholar] [CrossRef]
- Cunningham Jr, F.X.; Lee, H.; Gantt, E. Carotenoid biosynthesis in the primitive red alga Cyanidioschyzon merolae. Eukaryot. Cell 2007, 6, 533–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, N.; García-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid composition of marine red algae 1. J. Phycol. 2006, 42, 1208–1216. [Google Scholar] [CrossRef]
- Goss, R.; Jakob, T. Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth. Res. 2010, 106, 103–122. [Google Scholar] [CrossRef]
- Bernard, K.; Moss, G.; Toth, G.; Weedon, B. Absolute configuration of fucoxanthin. Tetrahedron Lett. 1976, 17, 115–118. [Google Scholar] [CrossRef]
- Dambek, M.; Eilers, U.; Breitenbach, J.; Steiger, S.; Büchel, C.; Sandmann, G. Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathway genes in Phaeodactylum tricornutum. J. Exp. Bot. 2012, 63, 5607–5612. [Google Scholar] [CrossRef]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef] [Green Version]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: Formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef]
- Airanthi, M.W.-A.; Sasaki, N.; Iwasaki, S.; Baba, N.; Abe, M.; Hosokawa, M.; Miyashita, K. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J. Agric. Food Chem. 2011, 59, 4156–4163. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hosokawa, M.; Matsukawa, N.; Hagio, M.; Shinoki, A.; Nishimukai, M.; Miyashita, K.; Yajima, T.; Hara, H. Suppressive effects of the marine carotenoids, fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur. J. Nutr. 2010, 49, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Yonekura, L.; Nagao, A. Low bioavailability of dietary epoxyxanthophylls in humans. Br. J. Nutr. 2008, 100, 273–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wu, H.; Wen, H.; Fang, H.; Hong, Z.; Yi, R.; Liu, R. Simultaneous determination of fucoxanthin and its deacetylated metabolite fucoxanthinol in rat plasma by liquid chromatography-tandem mass spectrometry. Mar. Drugs 2015, 13, 6521–6536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, Y.; Li, X.; Mei, Z.; Wu, S.; He, Y.; Jiang, X.; Sun, J.; Xiao, J.; Deng, L. Nanoencapsulation of cyanidin-3-O-glucoside enhances protection against UVB-induced epidermal damage through regulation of p53-mediated apoptosis in mice. J. Agric. Food Chem. 2018, 66, 5359–5367. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Zou, Y.; Liang, X.; Peng, Y.; McClements, D.J.; Hu, K. Encapsulation of resveratrol in zein/pectin core-shell nanoparticles: Stability, bioaccessibility, and antioxidant capacity after simulated gastrointestinal digestion. Food Hydrocoll. 2019, 93, 261–269. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, X.; McClements, D.J.; Zou, L.; Liu, X.; Liu, F. Co-encapsulation of epigallocatechin gallate (EGCG) and curcumin by two proteins-based nanoparticles: Role of EGCG. J. Agric. Food Chem. 2019, 67, 13228–13236. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and In Vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Guo, B.; Oliviero, T.; Fogliano, V.; Ma, Y.; Chen, F.; Capuano, E. Gastrointestinal bioaccessibility and colonic fermentation of fucoxanthin from the extract of the microalga Nitzschia laevis. J. Agric. Food Chem. 2019, 68, 1844–1850. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Xu, Y.; Zhao, L.; Yan, H.; Wang, S.; Wang, D. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers. Rsc Adv. 2018, 8, 35139–35149. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, Q.; Huang, L.; Chen, Z.; Zou, C.; Ma, Y.; Cao, M.-J.; Liu, G.-M.; Liu, Y.; Wang, Y. Fabricating hydrophilic particles with oleic acid and bovine serum albumin to improve the dispersibility and bioaccessibility of fucoxanthin in water. Food Hydrocoll. 2021, 118, 106752. [Google Scholar] [CrossRef]
- Guo, B.; Yang, B.; Pang, X.; Chen, T.; Chen, F.; Cheng, K.-W. Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food Funct. 2019, 10, 5644–5655. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Sun, X.; Wang, S.; Xu, Y. Fucoxanthin Isolated from Undaria pinnatifida Can Interact with Escherichia coli and lactobacilli in the Intestine and Inhibit the Growth of Pathogenic Bacteria. J. Ocean. Univ. China 2019, 18, 926–932. [Google Scholar] [CrossRef]
- Kiefer, C.; Sumser, E.; Wernet, M.F.; Von Lintig, J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc. Natl. Acad. Sci. USA 2002, 99, 10581–10586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. Evid. Based Complementary Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.H.T.; Bischoff, S.C. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities In Vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Gao, L.; Zhao, X. Rapid Purification of Fucoxanthin from Phaeodactylum tricornutum. Molecules 2022, 27, 3189. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin and rosmarinic acid combination has anti-inflammatory effects through regulation of NLRP3 inflammasome in UVB-exposed HaCaT keratinocytes. Mar. Drugs 2019, 17, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, Y.-F.; Chen, H.-Y.; Chang, Y.-J.; Shih, Y.-H.; Shieh, T.-M.; Wang, K.-L.; Hsia, S.-M. Protective effects of fucoxanthin on high glucose-and 4-hydroxynonenal (4-HNE)-induced injury in human retinal pigment epithelial cells. Antioxidants 2020, 9, 1176. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-containing cream prevents epidermal hyperplasia and UVB-induced skin erythema in mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Lu, Y.; Tao, T.; Wang, H.; Liu, G.-J.; Liu, X.-Z.; Liu, C.; Xia, D.-Y.; Hang, C.-H.; Li, W. Fucoxanthin mitigates subarachnoid hemorrhage-induced oxidative damage via sirtuin 1-dependent pathway. Mol. Neurobiol. 2020, 57, 5286–5298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Fan, Y.; Gao, Y.; Li, X.; Hu, Z.; Ding, K.; Wang, Y.; Wang, X. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Sci. Rep. 2017, 7, 46763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshii, H.; Yoshii, Y.; Asai, T.; Furukawa, T.; Takaichi, S.; Fujibayashi, Y. Photo-excitation of carotenoids causes cytotoxicity via singlet oxygen production. Biochem. Biophys. Res. Commun. 2012, 417, 640–645. [Google Scholar] [CrossRef] [Green Version]
- Guvatova, Z.; Dalina, A.; Marusich, E.; Pudova, E.; Snezhkina, A.; Krasnov, G.; Kudryavtseva, A.; Leonov, S.; Moskalev, A. Protective effects of carotenoid fucoxanthin in fibroblasts cellular senescence. Mech. Ageing Dev. 2020, 189, 111260. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.C.; Yusoff, F.M.; Imam, M.U.; Foo, J.B.; Ismail, N.; Azmi, N.H.; Tor, Y.S.; Khong, N.M.; Ismail, M. Increased fucoxanthin in Chaetoceros calcitrans extract exacerbates apoptosis in liver cancer cells via multiple targeted cellular pathways. Biotechnol. Rep. 2019, 21, e00296. [Google Scholar] [CrossRef]
- Kong, Z.-L.; Sudirman, S.; Hsu, Y.-C.; Su, C.-Y.; Kuo, H.-P. Fucoxanthin-rich brown algae extract improves male reproductive function on streptozotocin-nicotinamide-induced diabetic rat model. Int. J. Mol. Sci. 2019, 20, 4485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-L.; Chiu, Y.-T.; Hu, M.-L. Fucoxanthin enhances HO-1 and NQO1 expression in murine hepatic BNL CL. 2 cells through activation of the Nrf2/ARE system partially by its pro-oxidant activity. J. Agric. Food Chem. 2011, 59, 11344–11351. [Google Scholar] [PubMed]
- Zheng, J.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Cha, J.W.; Hyun, J.W. Fucoxanthin enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2014, 12, 4214–4230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Tian, X.; Zhang, W.; Zheng, P.; Huang, F.; Ding, G.; Yang, Z. Protective effects of fucoxanthin against alcoholic liver injury by activation of Nrf2-mediated antioxidant defense and inhibition of TLR4-mediated inflammation. Mar. Drugs 2019, 17, 552. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-B.; Kang, H.; Li, Y.; Park, Y.-K.; Lee, J.-Y. Fucoxanthin inhibits lipopolysaccharide-induced inflammation and oxidative stress by activating nuclear factor E2-related factor 2 via the phosphatidylinositol 3-kinase/AKT pathway in macrophages. Eur. J. Nutr. 2021, 60, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jeon, J.; Lee, J.S. Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phytother. Res. 2014, 28, 137–143. [Google Scholar] [CrossRef]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Phan, N.N.; Lu, W.-J.; Hieu, B.T.N.; Lin, Y.-C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-N.; Heo, S.-J.; Yoon, W.-J.; Kang, S.-M.; Ahn, G.; Yi, T.-H.; Jeon, Y.-J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef]
- Kawashima, T. A marine carotenoid, fucoxanthin, induces regulatory T cells and inhibits Th17 cell differentiation In Vitro. Biosci. Biotechnol. Biochem. 2011, 75, 2066–2069. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S. Obesity Rates Likely to Double by 2030 with Highest Rises in Lower-Income Countries. 2022. Available online: https://www.theguardian.com/global-development/2022/mar/04/obesity-rates-likely-to-double-by-2030-with-highest-rises-in-lower-income-countries (accessed on 20 December 2021).
- Wan-Loy, C.; Siew-Moi, P. Marine algae as a potential source for anti-obesity agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef] [Green Version]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen™ in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Gille, A.; Stojnic, B.; Derwenskus, F.; Trautmann, A.; Schmid-Staiger, U.; Posten, C.; Briviba, K.; Palou, A.; Bonet, M.L.; Ribot, J. A lipophilic fucoxanthin-rich Phaeodactylum tricornutum extract ameliorates effects of diet-induced obesity in C57BL/6J mice. Nutrients 2019, 11, 796. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef]
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, metabolite of fucoxanthin, improves obesity-induced inflammation in adipocyte cells. Mar. Drugs 2015, 13, 4799–4813. [Google Scholar] [CrossRef] [Green Version]
- Okada, T.; Mizuno, Y.; Sibayama, S.; Hosokawa, M.; Miyashita, K. Antiobesity effects of Undaria lipid capsules prepared with scallop phospholipids. J. Food Sci. 2011, 76, H2–H6. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Locke, R.M. Thermogenic mechanisms in brown fat. Physiol. Rev. 1984, 64, 1–64. [Google Scholar] [CrossRef] [Green Version]
- Mikami, N.; Hosokawa, M.; Miyashita, K.; Sohma, H.; Ito, Y.M.; Kokai, Y. Reduction of HbA1c levels by fucoxanthin-enriched akamoku oil possibly involves the thrifty allele of uncoupling protein 1 (UCP1): A randomised controlled trial in normal-weight and obese Japanese adults. J. Nutr. Sci. 2017, 6, e5. [Google Scholar] [CrossRef] [Green Version]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.-E. Undaria pinnatifida and fucoxanthin ameliorate lipogenesis and markers of both inflammation and cardiovascular dysfunction in an animal model of diet-induced obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef] [Green Version]
- Beppu, F.; Hosokawa, M.; Yim, M.-J.; Shinoda, T.; Miyashita, K. Down-regulation of hepatic stearoyl-CoA desaturase-1 expression by fucoxanthin via leptin signaling in diabetic/obese KK-A y mice. Lipids 2013, 48, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-T.; Chou, H.-N.; Huang, C.-j. Dietary fucoxanthin increases metabolic rate and upregulated mRNA expressions of the PGC-1alpha network, mitochondrial biogenesis and fusion genes in white adipose tissues of mice. Mar. Drugs 2014, 12, 964–982. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-M.; Kim, S.-M.; Cho, D.-Y.; Park, S.-J.; Joo, N.-S. The effect of xanthigen on the expression of brown adipose tissue assessed by 18F-FDG PET. Yonsei Med. J. 2016, 57, 1038–1041. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-C.; Shih, P.-H.; Wang, W.; Wu, C.-H.; Hsia, S.-M.; Wang, H.-J.; Hwang, P.-A.; Wang, C.-Y.; Chen, S.-H.; Kuo, Y.-T. Inhibitory effects of high stability fucoxanthin on palmitic acid-induced lipid accumulation in human adipose-derived stem cells through modulation of long non-coding RNA. Food Funct. 2015, 6, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-I.; Ko, H.-C.; Shin, H.-S.; Kim, H.-M.; Hong, Y.-S.; Lee, N.-H.; Kim, S.-J. Fucoxanthin exerts differing effects on 3T3-L1 cells according to differentiation stage and inhibits glucose uptake in mature adipocytes. Biochem. Biophys. Res. Commun. 2011, 409, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-S.; Tsai, M.-L.; Badmaev, V.; Jimenez, M.; Ho, C.-T.; Pan, M.-H. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARγ and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J. Agric. Food Chem. 2012, 60, 1094–1101. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilous, R.; Donnelly, R.; Idris, I. Handbook of Diabetes; John Wiley & Sons: Chichester, UK, 2021. [Google Scholar]
- World Health Organization (WHO). Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 16 February 2022).
- Cruz, N.G.; Sousa, L.P.; Sousa, M.O.; Pietrani, N.T.; Fernandes, A.P.; Gomes, K.B. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 99, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.; Wilding, J. Obesity and diabetes. In Bailliere’s Clinical Endocrinology and Metabolism; Harcourt Publishers Ltd.: Liverpool, UK, 1999. [Google Scholar]
- Lin, H.-T.V.; Tsou, Y.-C.; Chen, Y.-T.; Lu, W.-J.; Hwang, P.-A. Effects of low-molecular-weight fucoidan and high stability fucoxanthin on glucose homeostasis, lipid metabolism, and liver function in a mouse model of type II diabetes. Mar. Drugs 2017, 15, 113. [Google Scholar] [CrossRef] [Green Version]
- Woo, M.-N.; Jeon, S.-M.; Kim, H.-J.; Lee, M.-K.; Shin, S.-K.; Shin, Y.C.; Park, Y.-B.; Choi, M.-S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef]
- Nishikawa, S.; Hosokawa, M.; Miyashita, K. Fucoxanthin promotes translocation and induction of glucose transporter 4 in skeletal muscles of diabetic/obese KK-Ay mice. Phytomedicine 2012, 19, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Lyon, C.J.; Hsueh, W.A. Effect of plasminogen activator inhibitor–1 in diabetes mellitus and cardiovascular disease. Am. J. Med. 2003, 115, 62–68. [Google Scholar] [CrossRef]
- Armoni, M.; Harel, C.; Karnieli, E. Transcriptional regulation of the GLUT4 gene: From PPAR-γ and FOXO1 to FFA and inflammation. Trends Endocrinol. Metab. 2007, 18, 100–107. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Hung, Y.-L.; Tsai, Y.-K.; Chien, S.-Y.; Kong, Z.-L. The brown seaweed Sargassum hemiphyllum exhibits α-amylase and α-glucosidase inhibitory activity and enhances insulin release in vitro. Cytotechnology 2015, 67, 653–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawee-Ai, A.; Kim, A.T.; Kim, S.M. Inhibitory activities of microalgal fucoxanthin against α-amylase, α-glucosidase, and glucose oxidase in 3T3-L1 cells linked to type 2 diabetes. J. Oceanol. Limnol. 2019, 37, 928–937. [Google Scholar] [CrossRef]
- Zaharudin, N.; Staerk, D.; Dragsted, L.O. Inhibition of α-glucosidase activity by selected edible seaweeds and fucoxanthin. Food Chem. 2019, 270, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Moheimanian, N.; Mirkhani, H.; Sohrabipour, J.; Jassbi, A.R. Inhibitory Potential of Six Brown Algae from the Persian Gulf on α-Glucosidase and In Vivo Antidiabetic Effect of Sirophysalis Trinodis. Iran. J. Med. Sci. 2022, 2–11. [Google Scholar] [CrossRef]
- Wang, P.-T.; Sudirman, S.; Hsieh, M.-C.; Hu, J.-Y.; Kong, Z.-L. Oral supplementation of fucoxanthin-rich brown algae extract ameliorates cisplatin-induced testicular damage in hamsters. Biomed. Pharmacother. 2020, 125, 109992. [Google Scholar] [CrossRef]
- Doğru, A. Cancer 2nd Leading Cause of Death Globally, Data Reveals. 2022. Available online: https://www.aa.com.tr/en/health/cancer-2nd-leading-cause-of-death-globally-data-reveals/2493204 (accessed on 11 March 2022).
- Gutschner, T.; Diederichs, S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012, 9, 703–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, T.-W.; Choi, H.-J.; Lee, J.-Y.; Jeong, H.-S.; Kim, C.-H.; Joo, M.; Choi, J.-Y.; Han, C.-W.; Kim, S.-Y.; Choi, J.-S. Marine algal fucoxanthin inhibits the metastatic potential of cancer cells. Biochem. Biophys. Res. Commun. 2013, 439, 580–585. [Google Scholar] [CrossRef]
- Li, X.; Huang, R.; Liu, K.; Li, M.; Luo, H.; Cui, L.; Huang, L.; Luo, L. Fucoxanthin attenuates LPS-induced acute lung injury via inhibition of the TLR4/MYD88 signaling axis. Aging 2021, 13, 2655. [Google Scholar] [CrossRef]
- Lopes, F.G.; Oliveira, K.A.; Lopes, R.G.; Poluceno, G.G.; Simioni, C.; Da Silva Pescador, G.; Bauer, C.M.; Maraschin, M.; Derner, R.B.; Garcez, R.C. Anti-cancer effects of fucoxanthin on human glioblastoma cell line. Anticancer Res. 2020, 40, 6799–6815. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; Zhang, Y.; Wang, Z.; Yang, Y.; Bai, M.; Dai, Y. Fucoxanthin activates apoptosis via inhibition of PI3K/Akt/mTOR pathway and suppresses invasion and migration by restriction of p38-MMP-2/9 pathway in human glioblastoma cells. Neurochem. Res. 2016, 41, 2728–2751. [Google Scholar] [CrossRef]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Tanaka, T.; Miyamoto, S.; Mutoh, M. A marine bio-functional lipid, fucoxanthinol, attenuates human colorectal cancer stem-like cell tumorigenicity and sphere formation. J. Clin. Biochem. Nutr. 2017, 61, 16–112. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Mutoh, M. Induction of anoikis in human colorectal cancer cells by fucoxanthinol. Nutr. Cancer 2017, 69, 1043–1052. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. Rep. 2019, 23, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Li, Y.; White, W.L.; Lu, J. Extracts from New Zealand Undaria pinnatifida containing fucoxanthin as potential functional biomaterials against cancer in vitro. J. Funct. Biomater. 2014, 5, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Tafuku, S.; Ishikawa, C.; Yasumoto, T.; Mori, N. Anti-neoplastic effects of fucoxanthin and its deacetylated product, fucoxanthinol, on Burkitt’s and Hodgkin’s lymphoma cells. Oncol. Rep. 2012, 28, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Rokkaku, T.; Kimura, R.; Ishikawa, C.; Yasumoto, T.; Senba, M.; Kanaya, F.; Mori, N. Anticancer effects of marine carotenoids, fucoxanthin and its deacetylated product, fucoxanthinol, on osteosarcoma. Int. J. Oncol. 2013, 43, 1176–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zeng, Y.; Liu, Y.; Hu, X.; Li, S.; Wang, Y.; Li, L.; Lei, Z.; Zhang, Z. Fucoxanthin induces growth arrest and apoptosis in human bladder cancer T24 cells by up-regulation of p21 and down-regulation of mortalin. Acta Biochim. Biophys. Sin. 2014, 46, 877–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chen, S.; Xu, S.; Yu, X.; Ma, D.; Hu, X.; Cao, X. In Vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar. Drugs 2012, 10, 2055–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef]
- Ye, G.; Lu, Q.; Zhao, W.; Du, D.; Jin, L.; Liu, Y. Fucoxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway. Tumor Biol. 2014, 35, 11261–11267. [Google Scholar] [CrossRef]
- Liu, C.-L.; Lim, Y.-P.; Hu, M.-L. Fucoxanthin enhances cisplatin-induced cytotoxicity via NFκB-mediated pathway and downregulates DNA repair gene expression in human hepatoma HepG2 cells. Mar. Drugs 2013, 11, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell. Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.-X.; Yu, R.-T.; Liu, Z. Inhibition of two gastric cancer cell lines induced by fucoxanthin involves downregulation of Mcl-1 and STAT3. Hum. Cell 2018, 31, 50–63. [Google Scholar] [CrossRef]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine carotenoid fucoxanthin possesses anti-metastasis activity: Molecular evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.-X.; Hu, X.-M.; Xu, S.-Q.; Jiang, Z.-J.; Yang, W. Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway. Eur. J. Pharmacol. 2011, 657, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Barbosa, M.; Pereira, D.M.; Sousa-Pinto, I.; Valentão, P.; Azevedo, I.C.; Andrade, P.B. Chemical profiling of edible seaweed (Ochrophyta) extracts and assessment of their in vitro effects on cell-free enzyme systems and on the viability of glutamate-injured SH-SY5Y cells. Food Chem. Toxicol. 2018, 116, 196–206. [Google Scholar] [CrossRef]
- Yu, J.; Lin, J.-J.; Yu, R.; He, S.; Wang, Q.-W.; Cui, W.; Zhang, J.-R. Fucoxanthin prevents H2O2-induced neuronal apoptosis via concurrently activating the PI3-K/Akt cascade and inhibiting the ERK pathway. Food Nutr. Res. 2017, 61, 1304678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In Vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ1-42) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Fucoxanthin ameliorates inflammation and oxidative reponses in microglia. J. Agric. Food Chem. 2013, 61, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Liu, F.; Lin, J.; Chen, H.; Huang, C.; Chen, L.; Zhou, Y.; Ye, L.; Zhang, K.; Jin, J. Fucoxanthin inhibits β-amyloid assembly and attenuates β-amyloid oligomer-induced cognitive impairments. J. Agric. Food Chem. 2017, 65, 4092–4102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Kwon, S.-H.; Chun, Y.S.; Gu, M.-Y.; Yang, H.O. Anti-neuroinflammatory effects of fucoxanthin via inhibition of Akt/NF-κB and MAPKs/AP-1 pathways and activation of PKA/CREB pathway in lipopolysaccharide-activated BV-2 microglial cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H. Fucoxanthin, a marine carotenoid, attenuates β-amyloid oligomer-induced neurotoxicity possibly via regulating the PI3K/Akt and the ERK pathways in SH-SY5Y cells. Oxidative Med. Cell. Longev. 2017, 2017, 6792543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, a marine carotenoid, reverses scopolamine-induced cognitive impairments in mice and inhibits acetylcholinesterase In Vitro. Mar. Drugs 2016, 14, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Chen, W.; Tian, F.; Yuan, C.; Wang, H.; Yue, H. Neuroprotective role of fucoxanthin against cerebral ischemic/reperfusion injury through activation of Nrf2/HO-1 signaling. Biomed. Pharmacother. 2018, 106, 1484–1489. [Google Scholar] [CrossRef]
- Jung, H.A.; Ali, M.Y.; Choi, R.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Kinetics and molecular docking studies of fucosterol and fucoxanthin, BACE1 inhibitors from brown algae Undaria pinnatifida and Ecklonia stolonifera. Food Chem. Toxicol. 2016, 89, 104–111. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing fucoxanthin as a selective dopamine D3/D4 receptor agonist: Relevance to Parkinson’s disease. Chem. Biol. Interact. 2019, 310, 108757. [Google Scholar] [CrossRef]
- Ma, S.Y.; Park, W.S.; Lee, D.-S.; Choi, G.; Yim, M.-J.; Lee, J.M.; Jung, W.-K.; Park, S.G.; Seo, S.-K.; Park, S.J. Fucoxanthin inhibits profibrotic protein expression In Vitro and attenuates bleomycin-induced lung fibrosis In Vivo. Eur. J. Pharmacol. 2017, 811, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, D.-S.; Park, S.K.; Choi, J.S.; Jung, W.-K.; Park, W.S.; Choi, I.-W. Fucoxanthin inhibits myofibroblast differentiation and extracellular matrix production in nasal polyp-derived fibroblasts via modulation of smad-dependent and smad-independent signaling pathways. Mar. Drugs 2018, 16, 323. [Google Scholar] [CrossRef] [Green Version]
- Šudomová, M.; Shariati, M.A.; Echeverría, J.; Berindan-Neagoe, I.; Nabavi, S.M.; Hassan, S.T. A microbiological, toxicological, and biochemical study of the effects of fucoxanthin, a marine carotenoid, on Mycobacterium tuberculosis and the enzymes implicated in its cell wall: A link between mycobacterial infection and autoimmune diseases. Mar. Drugs 2019, 17, 641. [Google Scholar] [CrossRef] [Green Version]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.K.; Ren, R.; Hashimoto, T.; Kanazawa, K. Fucoxanthin induces apoptosis in osteoclast-like cells differentiated from RAW264. 7 cells. J. Agric. Food Chem. 2010, 58, 6090–6095. [Google Scholar] [CrossRef]
- Koyama, T. Extracts of marine algae show inhibitory activity against osteoclast differentiation. Adv. Food Nutr. Res. 2011, 64, 443–454. [Google Scholar]
- Walsh, P.J.; McGrath, S.; McKelvey, S.; Ford, L.; Sheldrake, G.; Clarke, S.A. The osteogenic potential of brown seaweed extracts. Mar. Drugs 2019, 17, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-C.; Cheng, C.-Y.; Liu, C.-T.; Sue, Y.-M.; Chen, T.-H.; Hsu, Y.-H.; Hwang, P.-A.; Chen, C.-H. Alleviative effect of fucoxanthin-containing extract from brown seaweed Laminaria japonica on renal tubular cell apoptosis through upregulating Na+/H+ exchanger NHE1 in chronic kidney disease mice. J. Ethnopharmacol. 2018, 224, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Cheng, C.-Y.; Liu, C.-T.; Sue, Y.-M.; Chen, T.-H.; Hsu, Y.-H.; Huang, N.-J.; Chen, C.-H. Combined protective effects of oligo-fucoidan, fucoxanthin, and L-carnitine on the kidneys of chronic kidney disease mice. Eur. J. Pharmacol. 2021, 892, 173708. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jin, L.; Zheng, D.; Tang, X.; Yang, J.; Fan, L.; Xie, X. Fucoxanthin alleviates oxidative stress through Akt/Sirt1/FoxO3α signaling to inhibit HG-induced renal fibrosis in GMCs. Mar. Drugs 2019, 17, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudlikar, R.R.; Sargsyan, D.; Li, W.; Wu, R.; Zheng, M.; Kong, A.-N. Epigenomic, Transcriptomic, and Protective Effect of Carotenoid Fucoxanthin in High Glucose-Induced Oxidative Stress in Mes13 Kidney Mesangial Cells. Chem. Res. Toxicol. 2021, 34, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Côme, M.; Blanckaert, V.; Chini Zittelli, G.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Mimouni, V.; Chénais, B. Effect of Carotenoids from Phaeodactylum tricornutum on Palmitate-Treated HepG2 Cells. Molecules 2020, 25, 2845. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Hosokawa, M.; Niwano, Y.; Miyashita, K. Effects of dietary fucoxanthin on cholesterol metabolism in diabetic/obese KK-A y mice. Lipids Health Dis. 2012, 11, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, S.C.; Khong, N.M.; Yusoff, F.M. Physicochemical, microstructure and antioxidant properties of microalgae-derived fucoxanthin rich microcapsules. Algal Res. 2020, 51, 102061. [Google Scholar] [CrossRef]
- Lim, M.W.S.; Tan, K.M.; Chew, L.Y.; Kong, K.W.; Yan, S.W. Application of two-level full factorial design for the extraction of fucoxanthin and antioxidant activities from Sargassum siliquosum and Sargassum polycystum. J. Aquat. Food Prod. Technol. 2018, 27, 446–463. [Google Scholar] [CrossRef]
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A promising compound for human inflammation-related diseases. Life Sci. 2020, 255, 117850. [Google Scholar]
- Venugopal, V. Marine Polysaccharides: Food Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Miyashita, K.; Hosokawa, M. Fucoxanthin in the management of obesity and its related disorders. J. Funct. Foods 2017, 36, 195–202. [Google Scholar] [CrossRef]
- Bresson, J.-L.; Flynn, A.; Heinonen, M.; Hulshof, K.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; Moseley, B. Scientific opinion on the substantiation of health claims related to Undaria pinnatifida (Harvey) Suringar and maintenance or achievement of a normal body weight (ID 2345) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006: Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies. EFSA J. 2009, 7, 1–12. [Google Scholar]
- Koo, S.Y.; Mok, I.-K.; Pan, C.-H.; Kim, S.M. Preparation of fucoxanthin-loaded nanoparticles composed of casein and chitosan with improved fucoxanthin bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435. [Google Scholar] [CrossRef]
- Oryza Oil and Fat Chemical Co. Ltd. Fucoxanthin: Dietary Ingredient for Prevention of Metabolic Syndrome, Antioxidation and Cosmetics. Available online: http://www.oryza.co.jp/pdf/english/Fucoxanthin_1.0.pdf (accessed on 1 February 2022).
- Zahrah, Z.; Amin, M.; Alamsjah, M. The effect of fucoxanthin as coloring agent on the quality of Shrimp Paste. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surabaya, Indonesia, 26 September 2019; p. 012079. [Google Scholar]
- Kartikaningsih, H.; Mufti, E.D.; Nurhanief, A.E. Fucoxanthin from brown seaweed Sargassum cristaefolium tea in acid pH. In AIP Conference Proceedings; AIP Publishing: Long Island, NY, USA, 2017; p. 030009. [Google Scholar]
- Robertson, R.C.; Mateo, M.R.G.; O’Grady, M.N.; Guihéneuf, F.; Stengel, D.B.; Ross, R.P.; Fitzgerald, G.F.; Kerry, J.P.; Stanton, C. An assessment of the techno-functional and sensory properties of yoghurt fortified with a lipid extract from the microalga Pavlova lutheri. Innov. Food Sci. Emerg. Technol. 2016, 37, 237–246. [Google Scholar] [CrossRef]
- Mok, I.-K.; Yoon, J.-R.; Pan, C.-H.; Kim, S.M. Development, quantification, method validation, and stability study of a novel fucoxanthin-fortified milk. J. Agric. Food Chem. 2016, 64, 6196–6202. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.N. Cellular and In-Vitro Models to Assess Antioxidant Activities of Seaweed Extracts and the Potential Use of the Extracts as Ingredients; University College Cork: Cork, Ireland, 2013. [Google Scholar]
- Sugimura, R.; Suda, M.; Sho, A.; Takahashi, T.; Sashima, T.; Abe, M.; Hosokawa, M.; Miyashita, K. Stability of fucoxanthin in dried undaria pinnatifida (wakame) and baked products (scones) containing wakame powder. Food Sci. Technol. Res. 2012, 18, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Sasaki, K.; Ishihara, K.; Oyamada, C.; Sato, A.; Fukushi, A.; Arakane, T.; Motoyama, M.; Yamazaki, M.; Mitsumoto, M. Effects of fucoxanthin addition to ground chicken breast meat on lipid and colour stability during chilled storage, before and after cooking. Asian-Australas. J. Anim. Sci. 2008, 21, 1067–1072. [Google Scholar] [CrossRef]
- Atma, Y. Studi penggunaan angkak sebagai pewarna alami dalam pengolahan sosis daging sapi. J. Teknol. 2015, 7, 76–85. [Google Scholar]
- Wang, X.; Li, H.; Wang, F.; Xia, G.; Liu, H.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X. Isolation of fucoxanthin from Sargassum thunbergii and preparation of microcapsules based on palm stearin solid lipid core. Front. Mater. Sci. 2017, 11, 66–74. [Google Scholar] [CrossRef]
- Mok, I.-K.; Lee, J.K.; Kim, J.H.; Pan, C.-H.; Kim, S.M. Fucoxanthin bioavailability from fucoxanthin-fortified milk: In Vivo and In Vitro study. Food Chem. 2018, 258, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. Cancer 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Ax, K.; Schubert, H. Stability of lycopene emulsions in food systems. J. Food Sci. 2003, 68, 2730–2734. [Google Scholar] [CrossRef]
- Nuñez de González, M.T.; Attaie, R.; Mora-Gutierrez, A.; Woldesenbet, S.; Jung, Y. Stability of fucoxanthin in pasteurized skim and whole goat milk. Foods 2021, 10, 1647. [Google Scholar] [CrossRef]




| Species | Fucoxanthin Content (mg/g Sample) | Sample Condition | Country Origin | Extraction Method; Solution | Detection Technique ** | References |
|---|---|---|---|---|---|---|
| Padina minor | 0.50 | Dried | Malaysia | Solvent/maceration;ethanol | HPTLC | [37] |
| Padina pavonica | 0.43 | |||||
| Sargassum polycystum | 0.41 | |||||
| Euchema cottoni | 0.94 | |||||
| Sargassum sp. | 1.50 | Dried | Malaysia | Solvent/maceration;methanol | RP-HPLC-DAD | [38] |
| Saccharina japonica | 0.07 | Dried | Malaysia | Solvent/maceration;methanol | HPLC-DAD | [39] |
| Sargassum siliquosum | 1.41 | Dried | Malaysia | Solvent/maceration;methanol | HPLC-UV | [40] |
| Sargassum polycystum | 0.31 | |||||
| Sargassum binderi | 7.4 | Dried | Malaysia | Solvent/maceration;methanol | HPLC-UV | [41] |
| Sargassum plagyophyllum | 0.71 | Dried | Malaysia | Solvent/maceration;acetone-methanol | RP-HPLC-DAD | [42] |
| Turbinaria turbinate | 0.59 | |||||
| Sargassum duplicatum | 0.146 | Dried | Indonesia | Solvent/maceration;methanol | TLC | [43] |
| Sargassum polycystum | 0.155 -0.587 * | Fresh | Indonesia | Ultrasound-assisted extraction (UAE); acetone | HPLC-UV | [44] |
| Turbinaria decurrens | 0.65 | Dried | Indonesia | Solvent/maceration;ethanol | HPLC-UV | [45] |
| Sargassum crassifolium J. Agard | 1.64 | Dried | Indonesia | Solvent/maceration;chloroform-methanol | HPLC-DAD | [46] |
| Padina australis Hauck | 1.29 | |||||
| Turbinaria ornata (Turner) J. Agardh | 1.27 | |||||
| Sphaerotrichia divaricata | 0.11–1.48 | Dried | Japan | Solvent/maceration;ethanol | HPLC-UV | [47] |
| Sargassum horneri (Turner) J. Agardh | 2.12 | Dried | Japan | Solvent/maceration;chloroform-methanol | HPLC-DAD | [46] |
| Cystoseira Hakodatensis (Yendo) Fensholt | 1.99 | |||||
| Sargassum siliquastrum (Mertens ex Turner) C. Agardh | 2.01 | |||||
| Ecklonia kurome Okamura | 1.68 | |||||
| Undaria pinnatifida | 0.39 | Fresh | Japan | Pressurized liquid extraction (PLE); liquefied dimethyl ether (DME) | HPLC-UV | [48] |
| Sargassum horneri (Turner) | 1.35–4.49 * | Dried | Japan | Solvent/maceration;chloroform-methanol | HPLC-DAD | [49] |
| Cystoseira hakodatensis (Yendo) | 0.63–4.14 * | |||||
| Hincksia mitchellae P.C. Silva | 5.50 | Dried | Taiwan | Soxhlet assisted extraction (SAE); ethyl acetate | HPLC | [50] |
| Saccharina japonica (Areschoug) C.E.Lane, C.Mayes, Druehl & G.W.Saunders | 0.45 | Dried | Korea | Soxhlet assisted extraction (SAE); n-hexane | HPLC | [51] |
| Sargassum horneri (Turner) C.Agardh | 0.77 | Dried | Korea | Supercritical fluid extraction (SFE); CO2-ethanol | HPLC-DAD | [52] |
| Sargassum japonica J.E. Areschoug | 0.41 | |||||
| Sargassum horneri | 0.28 | Fresh | China | Solvent/maceration;ethanol | HPLC-DAD | [53] |
| Sargassum fusiforme (Harvey) Setchell | 2.62 | Fresh | China | Solvent/maceration;acetone-ethanol | Spectro | [54] |
| Laminaria japonica Aresch | 0.03 | Fresh | China | Microwave-assisted extraction; Heptane-acetone-water | LC-ESI-MS, HPLC-UV, 1 H-NMR | [55] |
| Sargassum fusiforme (Harvey) Setchell | 0.01 | Dried | ||||
| Undaria pinnatifida (Harvey) Suringar | 0.73 | |||||
| Cystoseira indica | 0.77–0.81 | Dried | Iran | Ultrasound-assisted extraction (UAE); methanol (C. indica), methanol-acetone (S. angustifolium) | HPLC-DAD | [56] |
| Sargassum angustifolium | 0.70–0.79 | |||||
| Dictyota indica | 0.211–0.463 * | Dried | Iran | Solvent/maceration;methanol | HPLC-UV | [35] |
| Iyengaria Stellata | 0.026–0.055 * | |||||
| Padina tenuis | 0.018–0.043 * | |||||
| Colpomenia sinuosa | 0.014–0.019 * | |||||
| Nizamuddinia zanardinii (Schiffner) P.C.Silva | 0.81–1.65 * | Dried | Iran | Solvent/maceration;chloroform-methanol-water | RP-HPLC-DAD | [33] |
| Cystoseira indica (Thivy & Doshi) Mairh | 2.33–3.56 * | |||||
| Sargassum swartzii C. Agardh | 0.17 | Dried | India | Soxhlet assisted extraction (SAE); ethyl acetate | FTIR, 1 H-NMR, 13 C-NMR | [57] |
| Padina tetrastromatica | 0.75 | Dried | India | Ultrasonic-assisted extraction (UAE);ethanol | FTIR, HPLC-UV, Orbitrap-MS | [58] |
| Dictyopteris australis | 0.23 | Dried | India | Solvent/maceration;acetone | Spectro | [59] |
| Dictyota dichotoma | 0.18 | |||||
| Iyengaria stellate | 0.18 | |||||
| Lobophora variegata | 0.23 | |||||
| Padina gymnospora | 0.43 | |||||
| Padina tetrastromatica | 0.41 | |||||
| Sargassum linearifolium | 0.37 | |||||
| Spatoglossum asperum | 0.58 | |||||
| Stoechospermum marginatum | 0.37 | |||||
| Turbinaria spp. | 0.43 | |||||
| Fucus vesiculosus | 0.657 | Dried | Ireland | Solvent/maceration;water | HPLC-UV, LC-MS | [60] |
| Alaria esculenta | 0.822 | |||||
| Himanthalia elongata | 18.6 | Dried | Ireland | Solvent/maceration;hexane-diethyl ether-chloroform | LC-ESI-MS, HPLC-DAD, 1 H-NMR | [61] |
| Alaria esculenta | 0.87 | Fresh | Ireland | Solvent/maceration;acetone | HPLC-DAD | [62] |
| Fucus vesiculosus | 0.7 | |||||
| Laminaria digitata | 0.65 | |||||
| Fucus serratus | 3.57 | Dried | Ireland | Solvent/maceration;hexane-acetone | HPLC-DAD | [63] |
| Laminaria digitata | 1.403 | |||||
| Ascophyllum nodosum (Linnaeus) Le Jolis | 0.022 | Dried | Ireland | Vortex-assisted solid-liquid micro-extraction (VAE); ethanol | HPLC-PDA | [64] |
| Fucus vesiculosus Linnaeus | 0.02 | |||||
| Dictyota dichotoma (Hudson) J.V.Lamouroux | 0.60 | Dried | Portugal | Vortex-assisted solid-liquid micro-extraction (VAE); ethanol | HPLC-PDA | [64] |
| Sargassum vulgare C. Agardh | 0.40 | |||||
| Zonaria tournefortii (J.V.Lamouroux) Montagne | 0.80 | |||||
| Laminaria ochroleuca | 0.004–0.16 * | Dried | Portugal | Solvent/maceration;acetone | HPLC-DAD | [32] |
| Saccharina latissima | 0.02–0.12 * | |||||
| Saccorhiza polyschides | 0.08–0.24 * | |||||
| Sargassum muticum (Yendo) Fensholt | 0.55 | Dried | Spain | Supercritical fluid extraction (SFE); CO2-ethanol | HPLC-DAD | [65] |
| Cystoseira barbata (Stackh.) C. Agardh | 3.0 | Dried | Ukraine | Solvent/maceration;ethanol | TLC | [66] |
| Undaria pinnatifida | 0.70 | Dried | New Zealand | Solvent/maceration;ethanol | HPLC-DAD | [67] |
| Undaria pinnatifida | 1.77–2.08 * | Dried | New Zealand | Solvent/maceration;methanol | HPLC-DAD | [68] |
| 3.32–4.96 * | Fresh |
| Bioactivities | Mechanisms/Benefits | References |
|---|---|---|
| Antioxidant |
| [26,38,106,107,108,109,110,111,112,113,114,118,119,120,121,204,205] |
| Anti-inflammatory |
| [108,110,111,123,124,125,126] |
| Anti-obesity |
| [90,123,128,129,130,131,133,134,136,137,138,139,142,143] |
| Anti-diabetic |
| [123,130,132,149,150,151,152,155,156,157,186] |
| Anti-cancer |
| [112,163,164,165,166,167,168,169,170,171,172,176] |
| Neuroprotective |
| [113,149,180,181,182,183,184,185,186,188] |
| Antifibrotic |
| [191,192] |
| Antitubercular |
| [193] |
| Kidney protection |
| [198,199,200,201] |
| Liver protection |
| [111,202,203,206] |
| Food | Fucoxanthin Source | Type of Source | Sensory Acceptability | Fucoxanthin Total Lost | References |
|---|---|---|---|---|---|
| Shrimp paste | Sargassum sp. extract | Macroalgae | Favourable at 12% | N/A | [212] |
| Fortified skimmed and whole milk | Food grade fucoxanthin | N/A | N/A | 4% | [64] |
| Dried tea | Sargassum cristaefolium extract | Macroalgae | N/A | N/A | [213] |
| Plain yogurt | Pavlova lutheri lipid extract | Microalgae | Not acceptable | N/A | [214] |
| Fortified skimmed and whole milk | Phaeodactylum tricornutum extract | Macroalgae | N/A | 9% | [215] |
| Yogurt | Fucus vesiculosus and Ascophyllum nodosum extract | Macroalgae | Favourable at 0.5%; water extract of A. nodosum | 0% | [216] |
| Fluid milk | Fucus vesiculosus and Ascophyllum nodosum extract | Macroalgae | Not acceptable | 0% | [216] |
| Scones | Undaria pinnatifida powder | Macroalgae | Favourable at 0.5 and 2% | <15% | [217] |
| Semolina wheat-based pasta | Undaria pinnatifida powder | Macroalgae | Favourable at 10% | <10% | [218] |
| Ground chicken breast meat | Undaria pinnatifida extract | Macroalgae | N/A | N/A | [219] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Din, N.A.S.; Mohd Alayudin, ‘A.S.; Sofian-Seng, N.-S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. https://doi.org/10.3390/foods11152235
Din NAS, Mohd Alayudin ‘AS, Sofian-Seng N-S, Rahman HA, Mohd Razali NS, Lim SJ, Wan Mustapha WA. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods. 2022; 11(15):2235. https://doi.org/10.3390/foods11152235
Chicago/Turabian StyleDin, Nur Akmal Solehah, ‘Ain Sajda Mohd Alayudin, Noor-Soffalina Sofian-Seng, Hafeedza Abdul Rahman, Noorul Syuhada Mohd Razali, Seng Joe Lim, and Wan Aida Wan Mustapha. 2022. "Brown Algae as Functional Food Source of Fucoxanthin: A Review" Foods 11, no. 15: 2235. https://doi.org/10.3390/foods11152235
APA StyleDin, N. A. S., Mohd Alayudin, ‘A. S., Sofian-Seng, N. -S., Rahman, H. A., Mohd Razali, N. S., Lim, S. J., & Wan Mustapha, W. A. (2022). Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods, 11(15), 2235. https://doi.org/10.3390/foods11152235