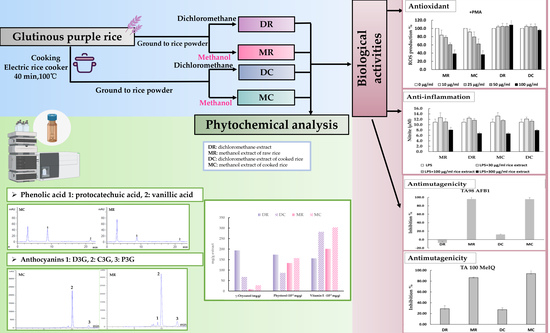
Phytochemical Profile and Chemopreventive Properties of Cooked Glutinous Purple Rice Extracts Using Cell-Based Assays and Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Purple Rice Extracts
2.3. Phytochemical Analysis
2.3.1. Spectrophotometric Determination of Phenolic Compounds
2.3.2. HPLC Analysis of Phenolic Compounds and γ-Oryzanol
2.3.3. Determination of Phytosterols and Tocols
2.3.4. GC–MS Analysis of Hydrophobic Components
2.4. Antioxidant Activity in Peripheral Blood Mononuclear Cells
2.5. Anti-Inflammatory Activity in Murine Macrophage Cells
2.6. Mutagenic and Antimutagenic Activities in Salmonella typhimurium
2.7. Clastogenic Activity in Rats
2.8. Determination of Xenobiotic Metabolizing Enzyme Activities in Rat Livers
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Constituents in Glutinous Purple Rice
3.2. Antioxidant Activity of Glutinous Purple Rice Extracts
3.3. Anti-inflammatory Activity of Glutinous Purple Rice Extracts
3.4. Mutagenicity and Antimutagenicity of Glutinous Purple Rice Extracts
3.5. Clastogenicity and Cytochrome P450 Activities of Methanol Extracts of Glutinous Purple Rice in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, Y.; Bao, J. Polyphenols in whole rice grain: Genetic diversity and health benefits. Food Chem. 2015, 180, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Gondal, T.A.; Keast, R.S.; Shellie, R.A.; Jadhav, S.R.; Gamlath, S.; Mohebbi, M.; Liem, D.G. Consumer acceptance of brown and white rice varieties. Foods 2021, 10, 1950. [Google Scholar] [CrossRef] [PubMed]
- Mbanjo, E.G.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The genetic basis and nutritional benefits of pigmented rice grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Min, B.; McClung, A.M.; Chen, M.H. Phytochemicals and antioxidant capacities in rice brans of different color. J. Food Sci. 2011, 76, C117–C126. [Google Scholar] [CrossRef]
- Chen, M.H.; McClung, A.M.; Bergman, C.J. Phenolic content, anthocyanins and antiradical capacity of diverse purple bran rice genotypes as compared to other bran colors. J. Cereal Sci. 2017, 77, 110–119. [Google Scholar] [CrossRef]
- Fracassetti, D.; Pozzoli, C.; Vitalini, S.; Tirelli, A.; Iriti, M. Impact of cooking on bioactive compounds and antioxidant activity of pigmented rice cultivars. Foods 2020, 9, 967. [Google Scholar] [CrossRef]
- Zaupa, M.; Calani, L.; Del Rio, D.; Brighenti, F.; Pellegrini, N. Characterization of total antioxidant capacity and (poly)phenolic compounds of differently pigmented rice varieties and their changes during domestic cooking. Food Chem. 2015, 187, 338–347. [Google Scholar] [CrossRef]
- Dokkaew, A.; Punvittayagul, C.; Insuan, O.; Wongpoomchai, R. Protective effects of defatted sticky rice bran extracts on the early stages of hepatocarcinogenesis in rats. Molecules 2019, 24, 2142. [Google Scholar] [CrossRef] [Green Version]
- Punvittayagul, C.; Sringarm, K.; Chaiyasut, C.; Wongpoomchai, R. Mutagenicity and antimutagenicity of hydrophilic and lipophilic extracts of Thai northern purple rice. Asian Pac. J. Cancer Prev. 2014, 15, 9517–9522. [Google Scholar] [CrossRef] [Green Version]
- Wongwichai, T.; Teeyakasem, P.; Pruksakorn, D.; Kongtawelert, P.; Pothacharoen, P.J.B. Pharmacotherapy. Anthocyanins and metabolites from purple rice inhibit IL-1β-induced matrix metalloproteinases expression in human articular chondrocytes through the NF-κB and ERK/MAPK pathway. Biomed. Pharmacother. 2019, 112, 108610. [Google Scholar] [CrossRef]
- Insuan, O.; Chariyakornkul, A.; Rungrote, Y.; Wongpoomchai, R. Antimutagenic and antioxidant activities of Thai rice brans. J. Cancer Prev. 2017, 22, 89. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.B. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef] [Green Version]
- Stagos, D.; Amoutzias, G.D.; Matakos, A.; Spyrou, A.; Tsatsakis, A.M.; Kouretas, D. Chemoprevention of liver cancer by plant polyphenols. Food Chem. Toxicol. 2012, 50, 2155–2170. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, W.; Xu, B. From rice bag to table: Fate of phenolic chemical compositions and antioxidant activities in waxy and non-waxy black rice during home cooking. Food Chem. 2016, 191, 81–90. [Google Scholar] [CrossRef]
- Thuengtung, S.; Ogawa, Y. Comparative study of conventional steam cooking and microwave cooking on cooked pigmented rice texture and their phenolic antioxidant. Food Sci. Nutr. 2020, 8, 965–972. [Google Scholar] [CrossRef]
- Chariyakornkul, A.; Punvittayagul, C.; Taya, S.; Wongpoomchai, R. Inhibitory effect of purple rice husk extract on AFB1-induced micronucleus formation in rat liver through modulation of xenobiotic metabolizing enzymes. BMC Complementary Altern. Med. 2019, 19, 237. [Google Scholar] [CrossRef] [Green Version]
- Phannasorn, W.; Chariyakornkul, A.; Sookwong, P.; Wongpoomchai, R. Comparative studies on the hepatoprotective effect of white and coloured rice bran oil against acetaminophen-induced oxidative stress in mice through antioxidant- and xenobiotic-metabolizing systems. Oxid. Med. Cell. Longev. 2021, 2021, 5510230. [Google Scholar] [CrossRef]
- Caldefie-Chézet, F.; Fusillier, C.; Jarde, T.; Laroye, H.; Damez, M.; Vasson, M.P.; Guillot, J. Potential anti-inflammatory effects of Melaleuca alternifolia essential oil on human peripheral blood leukocytes. Phytother. Res. 2006, 20, 364–370. [Google Scholar] [CrossRef]
- Bhawamai, S.; Lin, S.H.; Hou, Y.Y.; Chen, Y.H. Thermal cooking changes the profile of phenolic compounds but does not attenuate the anti inflammatory activities of black rice. Food Nutr. Res. 2016, 60, 32941. [Google Scholar] [CrossRef]
- Nilnumkhum, A.; Punvittayagul, C.; Chariyakornkul, A.; Wongpoomchai, R. Effects of hydrophilic compounds in purple rice husk on AFB1-induced mutagenesis. Mol. Cell. Toxicol. 2017, 13, 171–178. [Google Scholar] [CrossRef]
- Guideline, I.H.T. Guidance on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals M3 (R2). In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 11 June 2009. [Google Scholar]
- Kittichaiworakul, R.; Taya, S.; Chariyakornkul, A.; Chaiyaso, T.; Wongpoomchai, R. Antigenotoxic effects and possible mechanism of red yeast (Sporidiobolus pararoseus) on Aflatoxin B1-induced mutagenesis. Biomolecules 2021, 11, 734. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Chariyakornkul, A.; Jarukamjorn, K.; Wongpoomchai, R. Protective role of vanillic acid against diethylnitrosamine- and 1,2-dimethylhydrazine-induced hepatocarcinogenesis in rats. Molecules 2021, 26, 2718. [Google Scholar] [CrossRef]
- Luzhna, L.; Kathiria, P.; Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 2013, 4, 131. [Google Scholar] [CrossRef] [Green Version]
- Barros, A.I.; Nunes, F.M.; Gonçalves, B.; Bennett, R.N.; Silva, A.P. Effect of cooking on total vitamin C contents and antioxidant activity of sweet chestnuts (Castanea sativa Mill.). Food Chem. 2011, 128, 165–172. [Google Scholar] [CrossRef]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. Vitamins C and B and phenolic compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Chatthongpisut, R.; Schwartz, S.J.; Yongsawatdigul, J. Antioxidant activities and antiproliferative activity of Thai purple rice cooked by various methods on human colon cancer cells. Food Chem. 2015, 188, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hiemori, M.; Koh, E.; Mitchell, A.E. Influence of cooking on anthocyanins in black rice (Oryza sativa L. japonica var. SBR). J. Agric. Food Chem. 2009, 57, 14. [Google Scholar] [CrossRef]
- Sadilova, E.; Stintzing, F.; Carle, R. Thermal degradation of acylated and nonacylated anthocyanins. J. Food Sci. 2006, 71, C504–C512. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kumar, S.; Sehgal, A.; Singh, S.; Kumari, S.; Brisc, M.C.; Munteanu, M.A.; Brisc, C.; Buhas, C.L.; Judea-Pusta, C.; et al. Rice bran, an off-shoot to newer therapeutics in neurological disorders. Biomed. Pharmacother. 2021, 140, 111796. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A.; Dall’Asta, C.; Galaverna, G.; Scazzina, F.; Pellegrini, N. Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nutr. Food Res. 2007, 51, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.G.; Mathieu, D.; Loudet, C.; Buchoux, S.; Dufourc, E.J. Plant sterols in rafts: A better way to regulate membrane thermal shocks. FASEB J. 2007, 21, 1714–1723. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.C.; Huang, S.H.; Ng, L.T. Effects of cooking conditions on the concentrations of extractable tocopherols, tocotrienols and γ-oryzanol in brown rice: Longer cooking time increases the levels of extractable bioactive components. Eur. J. Lipid Sci. Technol. 2015, 117, 349–354. [Google Scholar] [CrossRef]
- Moreau, R.A.; Hicks, K.B.; Powell, M.J. Effect of heat pretreatment on the yield and composition of oil extracted from corn fiber. J. Agric. Food Chem. 1999, 47, 2869–2871. [Google Scholar] [CrossRef]
- Min, B.; McClung, A.; Chen, M.-H. Effects of hydrothermal processes on antioxidants in brown, purple and red bran whole grain rice (Oryza sativa L.). Food Chem. 2014, 159, 106–115. [Google Scholar] [CrossRef]
- Chen, M.-H.; Bergman, C.J. A rapid procedure for analysing rice bran tocopherol, tocotrienol and γ-oryzanol contents. J. Food Compos. Anal. 2005, 18, 139–151. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer. 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Koh, Y.C.; Ho, C.T.; Pan, M.H. Recent advances in cancer chemoprevention with phytochemicals. J. Food Drug Anal. 2020, 28, 14–37. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Yang, T.; Xu, Y.; Luo, Y.; Zhong, X.; Shi, L.; Hu, T.; Guo, T.; Nie, Y.; Luo, F.; et al. δ-Tocotrienol, isolated from rice Bran, exerts an anti-Inflammatory effect via MAPKs and PPARs signaling pathways in lipopolysaccharide-stimulated macrophages. Int. J. Mol. Sci. 2018, 19, 3022. [Google Scholar] [CrossRef] [Green Version]
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef]
- Dixon, K.; Kopras, E. Genetic alterations and DNA repair in human carcinogenesis. Semin. Cancer. Biol. 2004, 14, 441–448. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Boersma, M.G.; van der Woude, H.; Jeurissen, S.M.; Schutte, M.E.; Alink, G.M. Flavonoids and alkenylbenzenes: Mechanisms of mutagenic action and carcinogenic risk. Mutat. Res. 2005, 574, 124–138. [Google Scholar] [CrossRef]
- Resende, F.A.; Vilegas, W.; Dos Santos, L.C.; Varanda, E.A. Mutagenicity of flavonoids assayed by bacterial reverse mutation (Ames) test. Molecules 2012, 17, 5255–5268. [Google Scholar] [CrossRef]
- Okamoto, T. Safety of quercetin for clinical application (Review). Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini. Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Preissner, S.C.; Hoffmann, M.F.; Preissner, R.; Dunkel, M.; Gewiess, A.; Preissner, S. Polymorphic cytochrome P450 enzymes (CYPs) and their role in personalized therapy. PLoS ONE 2013, 8, e82562. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Methanol Extract of Cooked Rice | Methanol Extract of Raw Rice |
|---|---|---|
| Spectrophotometry (per g extract) | ||
| Total phenolics (mg GAE) | 132 ± 4.98 * | 189 ± 11.4 |
| Total flavonoids (mg CE) | 101 ± 12.4 * | 137 ± 10.7 |
| Total anthocyanins (mg) | 4.46 ± 0.55 * | 8.22 ± 0.03 |
| HPLC (mg per g extract) | ||
| Protocatechuic acid | 5.40 ± 0.02 * | 2.26 ± 0.00 |
| Vanillic acid | 1.18 ± 0.00 * | 0.75 ± 0.00 |
| Delphinidin-3-glucoside | ND | 3.73 ± 0.10 |
| Cyanidin-3-glucoside | 34.3 ± 0.50 * | 65.7 ± 0.78 |
| Peonidin-3-glucoside | 5.53 ± 0.59 * | 13.7 ± 0.31 |
| Compounds (Per Gram Extract) | Raw Rice | Cooked Rice | ||
|---|---|---|---|---|
| DCM | Methanol | DCM | Methanol | |
| Gamma-oryzanol (mg) | 194 ± 0.74 | 8.82 ± 0.21 | 67.5 ± 0.03 * | 27.4 ± 0.29 # |
| Cycloartenyl ferulate (mg) | 59.9 ± 0.07 | 2.12 ± 0.06 | 15.4 ± 0.10 | 6.52 ± 0.18 |
| 24-methylene cycloartanyl ferulate (mg) | 41.9 ± 0.20 | 1.23 ± 0.04 | 8.12 ± 0.02 | 3.31 ± 0.05 |
| Campesteryl ferulate (mg) | 28.3 ± 0.72 | 2.36 ± 0.28 | 15.7 ± 0.01 | 6.20 ± 0.06 |
| β-Sitosteryl ferulate (mg) | 63.4 ± 0.25 | 3.10 ± 0.16 | 28.3 ± 0.13 | 11.4 ± 0.12 |
| Total Phytosterols (μg) | 1735 ± 2.91 | 1337± 1.30 | 867 ± 7.62 * | 1573 ± 16.8 # |
| Stigmasterol + campesterol (μg) | 983 ± 4.02 | 519 ± 3.41 | 391 ± 8.78 | 573 ± 9.13 |
| β-sitosterol (μg) | 752 ± 2.35 | 818 ± 4.16 | 476 ± 4.61 | 1000 ± 8.70 |
| Total tocols (μg) | 156 ± 11.4 | 202 ± 3.75 | 283 ± 4.93 * | 303 ± 0.89 # |
| α-tocopherol (μg) | 12.8 ± 0.86 | 10.1 ± 1.23 | 25.0 ± 1.17 * | 27.2 ± 0.76 # |
| β-tocopherol (μg) | 8.61 ± 0.85 | 12.0 ± 0.51 | 9.28 ± 0.83 * | 17.4 ± 1.17 # |
| γ-tocopherol (μg) | 31.3 ± 2.41 | 14.7 ± 1.82 | 42.6 ± 2.05 * | 35.9 ± 2.50 # |
| δ-tocopherol (μg) | 3.79 ± 0.11 | 13.2 ± 0.97 | 2.13 ± 0.24 | 5.06 ± 0.51 |
| α-tocotrienol (μg) | 4.11 ± 0.05 | 11.6 ± 0.32 | 11.7 ± 0.40 * | 17.8 ± 0.50 # |
| γ-tocotrienol (μg) | 86.4 ± 6.96 | 123 ± 2.08 | 181 ± 1.50 * | 183 ± 0.40 # |
| δ-tocotrienol (μg) | 8.97 ± 0.42 | 16.6 ± 0.65 | 10.4 ± 0.43 * | 16.9 ± 0.49 # |
| Retention Time (min) | Identified Compound | Dichloromethane Extract | Methanol Extract | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Raw Rice | Cooked Rice | Raw Rice | Cooked Rice | ||||||
| Relative Content (%) | Quality (%) | Relative Content (%) | Quality (%) | Relative Content (%) | Quality (%) | Relative Content (%) | Quality (%) | ||
| 5.45 | 8-methyl-1-decene | 1.26 | 91.1 | 1.22 | 89.2 | - | - | ||
| 5.53 | hexadecane | - | 0.16 | 84.2 | - | - | |||
| 8.24 | heptacosane | - | 0.69 | 86.6 | - | - | |||
| 10.01 | hexadecanoic acid | 3.22 | 94.7 | 2.92 | 91.0 | 13.1 | 97.6 | 4.80 | 98.0 |
| 11.34 | 9,12-octadecadienoic acid | 9.74 | 95.7 | 10.7 | 93.0 | 34.8 | 96.1 | 16.6 | 97.4 |
| 11.38 | 1,19-eicosadiene | 23.7 | 93.9 | 16.9 | 94.7 | 29.3 | 92.5 | 16.2 | 92.4 |
| 14.21 | hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | - | - | 4.17 | 88.2 | 3.30 | 90.9 | ||
| 15.55 | z,e-2-methyl-3,13-octadecadien-1-ol | - | - | 4.84 | 84.8 | 10.4 | 91.4 | ||
| 16.63 | squalene | - | 3.12 | 85.6 | - | 1.93 | 94.0 | ||
| 22.78 | campesterol | - | - | - | 4.15 | 84.4 | |||
| 23.37 | stigmasterol | 7.75 | 85.1 | 4.31 | 86.5 | - | 10.5 | 92.0 | |
| 24.78 | β-sitosterol | 38.5 | 87.6 | 35.5 | 86.7 | 12.8 | 83.5 | 24.1 | 81.3 |
| 26.97 | cycloartenol acetate | 2.62 | 80.9 | 7.83 | 85.6 | - | 2.95 | 84.5 | |
| Total identification (%) | 86.8 | 83.3 | 98.9 | 94.9 | |||||
| Parameters | 5% Tween 80 | Methanol Extract | |
|---|---|---|---|
| Raw Rice | Cooked Rice | ||
| Micronucleated cells (/1000 hepatocytes) | 1.40 ± 0.66 | 0.83 ± 0.40 | 1.08 ± 0.73 |
| Binucleated cells (/1000 hepatocytes) | 7.81 ± 1.90 | 6.38 ± 0.74 | 5.90 ± 1.56 |
| Mitotic index (%) | 0.25 ± 0.60 | 0.00 ± 0.00 | 0.17 ± 0.26 |
| CYP1A1 activity (fmole/min/mg/protein) | 1.53 ± 0.91 | 1.56 ± 0.26 | 1.89 ± 1.20 |
| CYP1A2 activity (fmole/min/mg/protein) | 2.23 ± 1.32 | 2.07 ± 0.42 | 2.31 ± 1.02 |
| CYP3A2 activity (pmole/min/mg/protein) | 4.87 ± 1.15 | 3.74 ± 0.63 | 4.98 ± 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Chariyakornkul, A.; Phannasorn, W.; Mahatheeranont, S.; Wongpoomchai, R. Phytochemical Profile and Chemopreventive Properties of Cooked Glutinous Purple Rice Extracts Using Cell-Based Assays and Rat Model. Foods 2022, 11, 2333. https://doi.org/10.3390/foods11152333
Guo H, Chariyakornkul A, Phannasorn W, Mahatheeranont S, Wongpoomchai R. Phytochemical Profile and Chemopreventive Properties of Cooked Glutinous Purple Rice Extracts Using Cell-Based Assays and Rat Model. Foods. 2022; 11(15):2333. https://doi.org/10.3390/foods11152333
Chicago/Turabian StyleGuo, Huina, Arpamas Chariyakornkul, Warunyoo Phannasorn, Sugunya Mahatheeranont, and Rawiwan Wongpoomchai. 2022. "Phytochemical Profile and Chemopreventive Properties of Cooked Glutinous Purple Rice Extracts Using Cell-Based Assays and Rat Model" Foods 11, no. 15: 2333. https://doi.org/10.3390/foods11152333
APA StyleGuo, H., Chariyakornkul, A., Phannasorn, W., Mahatheeranont, S., & Wongpoomchai, R. (2022). Phytochemical Profile and Chemopreventive Properties of Cooked Glutinous Purple Rice Extracts Using Cell-Based Assays and Rat Model. Foods, 11(15), 2333. https://doi.org/10.3390/foods11152333