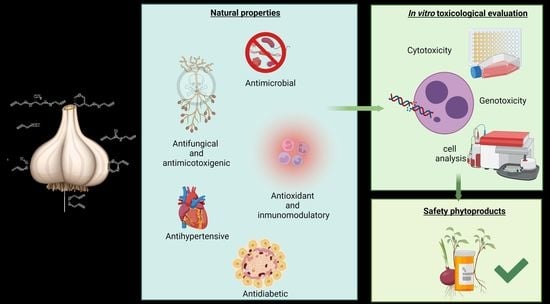
In Vitro Toxicity Studies of Bioactive Organosulfur Compounds from Allium spp. with Potential Application in the Agri-Food Industry: A Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility and Exclusion Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Extraction and Data Items
2.6. Risk of Bias in Individual Studies
2.7. Results Construction and Statistical Analysis
3. Results
3.1. Search Results
3.2. Study Characteristics and Results of Individual Studies
3.3. In Vitro Studies of OSCs from Allium spp. Focused on Safety Evaluation for Agri-Food Application
| Allium Products (Pure Compound or Extract) | Assays Performed | Experimental Model | Concentration Ranges and Time Exposure | Main Results | Reference |
|---|---|---|---|---|---|
| Ajoene Allicin | Survival by MTT assay Total cell protein | FS4 BHK21 BJA-B | 5–20 µg/mL for 48 h. | EC50(ajoene): FS4 (36 µM) > BHK21 (30 µM) > BJA-B (12 µM). ED50 (ajoene): FS4 (450 fmol/cell) > BHK21 (190 fmol/cell) > BJA-B (85 fmol/cell). ED50(allicin): FS4 (720 fmol/cell) > BHK21 (430 fmol/cell) > BJA-B (185 fmol/cell). | [57] |
| Ajoene | Metabolic activity by MTT assay Cell death by trypan blue assay Levels of GSH, GSSG and acidic aminoacids Glu and Asp | BJA-B cells | 173 and 82 nmol/mL for 0–6 h. 385 and 150 fmol/cell for 48 h. | In both experiments, ↓ cell viability in a dose and time dependent manner. In the first minutes after exposure, GSH decreased and GSSG increased. The further course strongly depended on the dose. The Glu pool showed an immediate increase and in a later stage decreased. The Asp pool showed the contrary pattern. | [58] |
| DDS DAS | Clonal survival Mass growth rate. Anchorage-Independent Growth. | Control and differentiated HT29 cells | 1–3 µg/mL DDS 24 h 100–300 µg/mL DAS 24 h after which the cells were incubated in fresh medium for 10–12 days. Cells were exposed to 24-h intervals for seven days. | Dq (concentration at which no cell killing occurs) 0.10 ± 0.03 µg/mL for DAS and was not found for DDS. D37 (dose required to reduce survival from 100% to 37%) are 2.93 ± 0.14 µg/mL for DAS and 164 ± 12 µg/mL for DDS. | [59] |
| DAS, DADS | Clonal survival Induction of chromosome aberrations Induction of SCEs | CHO | 100–600 µg/mL DAS 2–10 µg/mL DDS | Cytotoxicity DADS > DAS. Both compounds induced chromosome aberrations and SCEs (DADS > DAS) +S9: reduction of the induction of SCEs by both compounds, and enhanced the generation of aberrations by DADS. | [60] |
| DAS, DADS, DPS, DPDS | Ames test | Ames test: Salmonella typhimurium strains TA98 or TA100 | Ames test was performed with S9 and microsomes from DAS-, DADS-, DPS-, DPDS-treated rats (1 mmol/Kg) | DAS, DPS, DPDS: increased activation of BaP, CP, N-PiP and PhIP, while DADS only increased mutagenicity of PhIP. In contrast, some OCS inhibited the mutagenicity of different mutagens, while other enhanced it. | [61] |
| DAS, DADS, garlic extract | Cytotoxicity by MTT assay Western blot analysis of Bcl-2, Bax and p53 expression Northern blot analysis Apoptosis by acridine orange staining | p-53-wild type H460 and p-53-null type H1299 non-small cell lung cancer cells (NSCLC) | DAS and DADS (0–25 µM) and garlic extract (0–200 µg/mL) for 1 h | The cell growth was significantly inhibited by DAS and DADS and slightly inhibited by garlic extract. The OSCs compounds and garlic extract have apoptotic potential on lung cancer cells, and the mechanism was regulated through p53-dependent or p-53 independent related Bax/Bcl-2 dual pathway. | [62] |
| DADS | Cell viability and apoptosis by flow cytometry Oxidative stress (carbonylated proteins, MDA) Western blot analysis | SH-SY5Y | 50 µM for 12 and 24 h 50 µM up to 2 h | Blockage in G2/M phase DADS induced a ROS-mediated activation of JNK/c-Jun pathway in neuroblastoma cells, and this activation led to apoptosis. | [63] |
| DADS | Survival by MTT assay Apoptosis by flow cytometry Cell signals by western blot analysis of phosphorylated forms of GSK-3β and Akt, and p85a PI3K Free radical levels and membrane lipid peroxidation | N18D3 | 10–200 µM for 2 h. 10, 25 µM for 2 h pretreatment and treated with 100 µM H2O2 for 30 min. 25, 100 µM for 2 h treatment with and without post-treatment of 100 µM H2O2 for 30 min. | Cellular viability was not affected up to 25 µM DAS. ↑ apoptotic cells at 100 µM of treatment and at 25 µM of pretreatment versus H2O2 treatment in these cells. ↑ the expressions of p85a PI3K, phosphorylated Akt and phosphorylated GSK-3 in N18D3 cells pretreated with 25 µM (2 h) and subsequently exposed to 100 µM H2O2 (30 min). Treatment with 100 µM reduced these biomarkers in N18D3 cells. ↑ the levels of free radicals and membrane lipid peroxidation a concentration-dependent manner. | [64] |
| Allicin | Cell proliferation by MTT assay Morphological apoptosis DNA fragmentation assay Cell cycle by flow cytometry Caspase-3 determination Expression of Cyt c, Bax and AIF by Western blot analysis | AGS | 5–100 µg/mL for 6, 12, 24 and 48 h. | Allicin caused inhibition of cellular growth in a concentration- and time-dependent manner. DNA fragmentation and morphological changes (degeneration of neuritis, shrinkage of cell bodies and condensation of nuclei) in cells exposed to 5–20 µg/mL for 24 h. 45.2% apoptotic cells. ↑ in the sub-G1 DNA content. ↓ in the proportion in cells of S phase after exposure to 20 µg/mL of allicin for 24 h. Allicin results in the release of Cyt c and in increase of mitochondrial Bax protein level. Caspase-3 activation and cleavage of PARP were not detected. | [65] |
| Ajoene | MTS/PMS chromogenic assay Cell adhesion assay | B16/BL6 HT-29 A549 MDA-MB-231 PANC-1 SKBR-3 NIH 3T3 3T3/HER2 Splenocytes B16/BL6-LEC1 | 10–100 µM for 24 h 1–100 µM for 24 h | IC50(B16/BL6) = 18 µM IC50(HT-29) = 19 µM IC50(A549) = 41 µM IC50(MDA-MB-231) = 7 µM IC50(PANC-1) = 38 µM IC50(SKBR-3) = 19 µM IC50(NIH 3T) = 17 µM IC50(3T3/HER2) = 9 µM IC50(Splenocytes) ≥100 µM B16/BL6 29% inhibition at 10 µM | [66] |
| DAS, DADS, DATS | Cell viability by trypan blue exclusion assay Wright staining and ApopTag assay for apoptosis ROS production Intracellular free [Ca2+] by Fura-2 assay mRNA expression of β-actin, bax, bcl-2, calpastatin phosphorylation of stress kinases analysis Western Blot analysis analysis Mitocondrial membrane potential Caspase 3 and 9 activity GST activity | T98G U87MG | 100 µM DAS 100 µM DADS 25 µM DATS for 24 h | The three garlic compounds induced cytotoxic effects via ROS production, increase in endoplasmic reticulum (ER) stress, decrease mitochondrial membrane potential, activation of stress kinases and cysteine proteases. | [67] |
| Allicin | In vitro tubulin polymerization assay and image analysis | NIH-3T3 | 0.2–25 µM | Depolymerizing effect of allicin in a concentration-dependent manner until 25 µM. Disruptive effect of allicin increases with the duration of incubation. | [68] |
| Ajoene (≥98%) | Cell viability by MTS assay Apoptosis by flow cytometry Microscopic evaluation | B16F10 | 1, 5, 10 µmol/L for 24 h | 5 and 10 µmol/L ↓ cell viability and this cytotoxic effect was not prevented by the addition of mevalonate or GGPP. Ajoene (5 µmol/L) in combination with atorvastatin (0.1 mmol/L) or pravastatin (0.1 mmol/L) ↓ cell viability in a synergistic way. Apoptosis observed by diminution in cell volume, condensation of cytoplasm. | [69] |
| DADS | Cell viability by MTT assay Apoptosis by fluorescence microscopy and flow cytometry p-ERK and ERK protein levels by Western blot analysis | MCF-7 | 50–400 μmol/L for 24 h. 200 μmol/L for 6, 12, 24 and 48 h. | ↓ cellular viability and ↑ apoptosis in concentration-dependent manner. These effects were observed mainly from 200 μmol/L of treatment. Inhibition of ERK and activation of SAPK/JNK and p38. | [70] |
| DAS | Cell viability by trypan blue exclusion assay ALP and LDH assays ROS generation Apoptosis by flow cytometry analysis Cell cycle analysis DNA fragmentation analysis Immunoblot analysis of caspase-3, NF-κB, ERK-2 | Colo 320 DM | 5–75 µM for 0–24 h 50 µM for 6, 12 h 50 µM for 12 h 50 µM for 12 h | 50 µM ↓ cell viabilityALP and LDH decreased with time. ROS increased.~55% apoptosis. Cell cycle arrest at G2/M Oligonucleosomal-laddering, characteristic of apoptosis.Higher protein expression of caspase-3 and NF-κB and lower expression of ERK-2. | [71] |
| DADS | MTT assayApoptosis by flow cytometry, DNA fragmentation assay and morphology analysisWestern blot analysis of the expression of phosphor-MAPKs (ERK, p38) | HL-60 | 1.25–20 mg/L for 96 h 5–20 mg/L for 24 h 10 mg/L for 24 h | 10 mg/L ↓59.6% cell viability Apoptosis was evidenced in a concentration-dependent manner by different assays. Inhibition of ERK and activation of p38 | [72] |
| DATS | MTT assay Apoptosis by flow cytometry Expression of Bcl-2, Bax, Bcl-xL/Bcl-xS, Cyt c, caspase 9 and poly(ADP-ribose) polymerase by western blotting. Activity of caspase 3 | A375 M14 | 5–60 µM for 24, 48, 72 h Exposure to IC50 for 72 h: A375 11.7 µM and M14 14.1 µM IC50 for 24, 48, 72 h IC50 for 16, 24, 36, 48 h | IC50(A375) = 11.7 µM IC50(M14) = 14.1 µM ↑ percentage of apoptosis Reduced Bcl-2 and Bcl-xL expression Increase in caspase-3 activity with time | [73] |
| n-DADS s-DADS | MTT assay Cell cycle analysis Apoptosis by flow cytometry and by fluorescence microscopy after staining with Hoechst 33,258 | MCF-7 | 0.01–2.00 mmol/L 48 for 72 h. 0.01, 0.05, 0.25 mmol/L for 48 h. 0.05 mmol/L for 48 h, microscopy | s- and n-DADS present concentration- and time-dependent inhibitory effects and similar cytotoxicity in MCF-7 cells. Apoptosis from 0.01 mmol/L for 48 h. The percentages of cells in G0/G1-, S- and G2/Mphase did not differ from each other. | [74] |
| DATS | Cell viability by trypan blue assay ROS by fluorescence microscopy and flow cytometry Mitochondrial ROS levels assay Immunoblot analysis | MDA-MB-231 | 10–100 μM for 16 h 10–80 μM for 1 h 10, 50 μM for 1 h 10–100 μM for 16 h | Apoptotic cell death in concentration- and time-dependent manner was observed with cell shrinkage and cytoplasmic membrane blebbing. ↑ ROS with activation of ASK1 and a downstream signal transduction JNK (C-Jun N-terminal kinase)-Bim pathway at 50–80 μM. | [75] |
| DAS | Cell viability, cell cycle and apoptosis by PI staining by flow cytometric assay DNA damage by Comet assay and DAPI staining Flow cytometric assay for the production of Ca2+ and the level of mitochondrial membrane potential Western blot of apoptotic associated proteins Microarray assay | HeLa | 25–100 µM for 24 h 75 µM for 0–2 h 75 µM for 0–72 h 5 μM DAS for 24 h | DNA damage and fragmentation. Induced apoptosis and decreased the viability in concentration- and time-dependent manner. Induced cell cycle arrest increasing G0/G1 cell population and decreasing G2/M and S cell population. Decreased levels of mitochondrial membrane potential and promoted the levels of Ca2+ DAS promoted the levels of Fas, FasL and caspase-8, Bax, cytochrome c, Apaf-1, Bid, caspase-9 and -3. 28 genes were expressed at least by 2-fold compared with the untreated control cells. | [76] |
| DATS | Comet assay Apoptosis and ROS by flow cytometry Immunoblotting for phosphoolorect-ERK1/2 (P-ERK1/2 | PC-3 cells transfected with the plasmid encoding p66ShcS36A or an empty pcDNA3.1 vector | After 24 of transfection, cells were treated with DATS (0–40 µM) | DATS increased p66Shc phosphorylation at serine 36, which was abolished by JNK inhibitor, and DATS-induced ROS formation was abolished in cells expressing p66ShcS36A variant. In cells expressing this variant, DATS-induced Akt dephosphorilation was reduced. The signaling pathway with P66Shc could be indispensable for DATS-induced prostate cancer cell death by modulating the Akt activity and ROS generation. | [77] |
| DATS | ROS by flow cytometryProtein levels by ImmunoblottingLabile iron poolDNA damage by comet assay and microscopy | PC-3 | 40 µM for 4 h 40 µM for 12 h | DATS-mediated increase in labile iron pool is regulated by JNK1 but not JNK2. Ferritin degradation in PC-3 cells treated with DATS is controlled by JNK1. DATS-induced increase in ROS formation is JNK1-dependent. Iron is not involved in DATS-induced cell death. DATS-induced DNA damage is not ameliorated by iron chelation. | [78] |
| DATS | Cell viability by MTT assay Apoptosis by DAPI staining ROS and Mitochondrial membrane potential by flow cytometry Caspase-9 and -3 activities Apoptosis associated proteins by Western blotting | Primary colorectal cancer cells | 10–40 µM for 24 h 20 µM for 6,12 h 20 µM for 24 h | Viability inhibition in a concentration-dependent way. Apoptosis induction. Nuclear shrinkage/condensation and nuclear fragmentation. ROS production induction and decreased level of mitochondrial membrane potential. Activation of caspase 9 and 3. Increased protein levels of cytochrome c, caspase -9 and caspase-3. | [79] |
| DATS | Cell survival by sulforhodamine B assay ROS by flow cytometry Protein level by immunoblotting | PC-3 PNT1A | 40 µM for 24 h | PNT1A cells are more resistant to cytotoxic effects than PC-3 cells. In these cells, reduction of induced p66Shc hosphorylation and ferritin degradation, reduction Akt inactivation, and ROS generation was nearly abolished in PNT1A cells. | [80] |
| Allium sivasicum aqueous extract | Cytotoxicity by Trypan blue exclusion assay and MTT assay Apoptosis by flow cytometry | MCF-7 MDA-MB-468 MDA-MD231 | 10–100 µg/mL, 48 h MCF-7 21 ± 1.4 µg/mL MDA-MB-468 22 ± 1.4 µg/mL MDA-MB-231 24 ± 1.3 µg/mL (24 h for all) | IC50(MCF-7) = 21 ± 1.4 µg/mL IC50(MDA-MB-468) = 22 ± 1.4 µg/mL IC50(MDA-MB-231) = 24 ± 1.3 µg/mL ↑ percentage of apoptosis | [81] |
| S-Allylmercaptocyteine | Cell proliferation by [3H] thymidine incorporation assay DNA fragmentation assay Free SH groups Cell cycle by flow cytometry Cytotoxicity by MTS assay | HEL OCIM-1 | 0.02, 0.05, 0.1, 0.25 mM 24 h in HEL cells 0.05 or 0.1 mM for 2 days 0.1, 0.25, 0.5, 1 mM for 1,2, 3 days 0.25 mM for 6 h and 0.1 mM for 72 h 0.002–2 mM | Significant reduction in [3H] thymidine incorporation Signs of DNA fragmentation Initial increase of free SH groups followed by progressive decrease with extended incubation Accumulation of cells in G2/M phase OCIM-1 more sensitive. LD50 (HEL) = 0.1 mM and LD50 (OCIM-1) = 0.046 mM | [82] |
| Allicin | Cell proliferation by MTT assay Apoptosis and cell cycle by flow cytometry | SGG-7901 | Not revealed Apoptosis: 3 mg/l for 12, 24, 48 h Cell cycle: 3, 6, 12 mg/L for 24 and 48 h | Growth inhibition in a concentration-dependent manner Increased apoptosis Cell cycle arrest in G2/M | [83] |
| DADS | Cell viability by MTT assay Apoptosis by phase contrast microscopy and flow cytometry | ECA109 L02 | 10–60 µg/mL for 24 h 20–80 µg/mL for 24 h | Cell viability inhibition in a concentration-dependent manner in ECA109. Less toxic in L02 Membrane blebbing and formation of apoptotic bodies. Cellular shrinkage. Apoptosis induction in a concentration-dependent manner | [84] |
| DADS | Cell viability by MTT assay Cell cycle and apoptosis by flow cytometry PCR to investigate G2/M phase relative molecular pathway Protein expression by Western blot | ECA109 L02 | 10–60 µg/mL for 24–72 h 20–60 µg/mL for 24 h | Cell viability Inhibition in a concentration-dependent manner. Apoptosis induction in a concentration-dependent manner. G2/M phase arrest. Upregulated levels of p21 and p53 Protein levels of caspase-3 and cleaved caspase-3 upregulated in a concentration-dependent way. Induced apoptosis through upregulation of Bax mRNA, downregulation of Bcl-2 mRNA and a shift of Bax/Bcl-2 ratio. Expression levels of MEK1 and ERK1/2 did not change, but p-MEK1 and p- ERK1/2 decreased | [85] |
| PTSO | Ames test MN test MLA assay comet assays (with and without Endo III and FPG enzymes) | Salmonella typhimurium strains L5178Ytk+/- Caco-2 | 5–100 µM for the different assays, depending on the viability of the cells (Trypan blue exclusion test) | PTSO was not mutagenic in the Ames test, although it was weak mutagenic in the MLA assay after 24 of treatment (2.5–20.0 µM). The parent compound did not induce MN on mammalian cells, although in presence S9, induced positive results (20 µM). PTSO did not induce DNA breaks or oxidative damage in the comet assays. | [17] |
| DPS, DPDS, and mixtures | Cell viability by PC, NR, MTS ROS, GSH Morphology study Ames test | Caco-2 cells S. typhimurium strains | 0–200 µM for 2, 4, 8 h | No cytotoxicity or mutagenicity and no significant adverse effects were reported. ROS scavenger activity was observed for both compounds. | [16] |
| Allicin | Cell viability by MTT assay Apoptosis by Hoechst staining and flow cytometry Expression levels of apoptosis-associated proteins by western blotting | MGC-803 BGC-823 SGC-7901 | 0.5–10 µg/mL for 48 h 1 µg/mL for 12, 24 and 48 h 0.01–10 µg/mL for 48 h | Cell viability is affected in a concentration and time-dependent manner. Apoptosis induction Enhanced expression levels of cleaved caspase 3 | [86] |
| DAS, DADS, DATS | Cytotoxicity assay by cell counting kit-8Protein expression by western blottingCaspase-8 and 9 activityImmunofluorescence analysisLuciferase reporter assayRT-PCR | BC3BCBL1HBL6BC2Ramos DG75 | 1–50 µM for 24 h | DAS and DADS slightly decreased viability DAT: IC50(BC3) = 13.7 ± 0.8 IC50(BCBL1) = 15.5 ± 1.0 IC50(HBL6) = 17.7 ± 0.6 IC50(BC2) = 14.6 ± 0.4 IC50(Ramos) = 43.4 ± 1.4 IC50(DG75) = 48.0 ± 0.9 Apoptosis by activation of caspases Suppression of NF-κB signaling | [87] |
| PTS | Ames test MN assay MLA assay comet assays (with and without Endo III and FPG enzymes) | S. typhimurium strains for Ames test; L5178Ytk+/− cells for MN and MLA assays; and Caco 2 cells for comet tests | 0–280 µM for the different assays, depending on the viability of the cells (total protein, NRU, MTS) | Not mutagenic neither in the Ames test nor in MLA. Genotoxic effects were reported in the MN test at the highest concentration assayed (17.25 µM) without S9, and also its metabolites (+S9, from 20 µM). ↑ breaks damage on CaCO2 cells at the highest concentration tested (280 µM) but it did not induce oxidative DNA damage. | [55] |
| DATS | Cell viability by MTT assay Cell cycle and apoptosis by flow cytometry Protein expression by western blot Nuclear morphological changes ROS and MMP | AGS Chang liver cells | 0–50 µM for 0–24 h 50 µM, 0–24 h | Concentration- and time-dependent decrease of cell viability in AGS cells. No effect on Chang liver cells. In AGS cells DATS induced G2/M arrest and apoptosis by blocking cell cycle into G1 phase, mitotic arrest, caspase-dependent apoptosis, and ROS-dependent AMPK activation | [88] |
| DATS | Cell viability by trypan blue exclusion assay Clonogenic assay ROS Expression of DR4 and DR5 by flow cytometry Immunocytochemistry Apoptosis by flow cytometry Immunoblotting. | U87MG A172 U343 T98 G | 25–50 µM for 30 min 25 µM 24 h 5–50 µM for 24 h and 25 µM for 0–24 h | Up-regulated DR5 receptor expression, and enhanced TRAIL-induced apoptosis through the downregulation of anti-apoptotic protein Mcl-1 and the upregulation of DR5 receptors through actions on the ROS-induced-p53 | [89] |
| Allicin | Cytotoxicity by MTT assay Cell proliferation and colony formation assays Protein expression by western blot analysis Gene expression by RT-qPCR Caspase activity Morphology study Apoptosis by flow cytometry | U251 | 15–90 µg/mL for 24 h. 5–90 µg/mL for 24, 48, 72 h. 30, 60 µg/mL for 48 h 30, 60 µg/mL 30, 60 µg/mL, 24 h 30, 60 µg/mL, 48 h | Cytotoxic effect in a concentration-dependent manner and nuclear morphology changes in U251 cells. IC50 = 41.97 µg allicin/mL for 24 h. Increased apoptosis Morphological changes of apoptotic cells (condensation of chromatin, nuclear fragmentation) Proliferation inhibition ↑ caspase-3, -8 and -9 activities and Fas/FasL and Bax mRNA expression levels. ↓ Bcl-2 expression levels in a dose-dependent manner. ↑ the activation of both intrinsic and extrinsic apoptosis signaling pathways in U251 cells. | [90] |
| DAS | Cell viability by MTT assay The extend of lipid accumulation ROS by flow cytometry qRT-PCR of inflammatory genes | 3T3L1 RAW 264.7 | 100 mM ethanol and treated with 50–500 µM DAS for 24 and 48 h. | ↑ viability in ethanol-exposed 3T3L1 cells treated with 200–500 µM for 24 h and 50–500 µM for 48 h. ↓ ROS production, reduces expression of pro-inflammatory cytokines, and enhance anti-inflammatory cytokine production in ethanol-exposed 3T3L1 cells treated with 50–100 µM for 24 or 48 h. 100 µM for 24h ↑ expression of M2 phenotype- specific genes in ethanol-exposed RAW 264.7 cells. | [91] |
| Allicin | MTT assay Cell cycle by flow cytometry RT-PCR of cyclin D1, MMP-9 and RARβ | CD44+ CD117+ cells | CD44+: 4–32 µg allicin/mL or 8–125 µg ATRA/mL or 5 µg/mL of allicin during 4 h followed by 8–125 µg ATRA/mL. Total time of exposure 48 h. CD117+: 0.5–24 µg allicin/mL or 4–64 µg ATRA/mL or 5 µg/mL of allicin during 4 h followed by 4–64 µg ATRA/mL. Total time of exposure 48 h. IC50 for 48 h. | IC50 CD44+: allicin/ATRA (17.53 µg/mL) ˂ allicin (29.19 µg/mL) ˂ ATRA (37.43 µg/mL) IC50 CD117+: ATRA (8.09 µg/mL) ˂ allicin (10.75 µg/mL) ˂ allicin/ATRA (13.65 µg/mL) ↑ of cells at the G2/M and G0/G1 phases in the CD44+ and CD117+ cells, respectively. The combination treatment caused the inhibition of CD44+ and CD117+ melanoma cells at the S phases compared to ATRA alone. ↑ cyclin D1 mRNA expression by all treatments and reduction of MMP-9 mRNA expression by allicin treatment both CD44+ and CD117+ cells. ↑ mRNA level of RARβ expression by allicin/ATRA treatment in CD117+ cells. Increased MMP-9 gene expression by allicin/ATRA and ATRA treatments in CD44+ cells. Allicin reinforces the ATRA-mediated inhibitory effects on CD44+ and CD117+ melanoma cells | [92] |
| DADS | Cell viability by trypan blue assay SiRNA Immunoblotting assay Apoptosis by flow cytometry DNA fragmentation assay Caspase-3/7 activity assay | HCT116 DLD-1 HT29 SW620 FHC | 5–100 µM for 24 h. 0–25 µM 20 h + 50 ng/mL TRAIL for 4 h. | 0–10 µM caused ˂20% CRC cell deaths. DADS + TRAIL produced concentration-dependent decreased of % survival in SW620 cells, but not in FHC cells. 0–10 µM did not alter the expression of pro-apoptotic proteins (Bax and Bid) or antiapoptotic proteins (XIAP and olorecta) and Bcl-2 were down-regulated in CRC cell lines. | [93] |
| Polymer films of Allium cepa L. | Cell viability by MTT assayAmes testMN assay | HepG2 GM-07492 S. typhimurium strains | Eluates from HTP-films and W-HTP films containing onion pulp were used at different concentrations | Cytotoxicity: HTP > W-HTP. No induction of MN was observed in both type of films, although the HTP films showed signs of mutagenicity in the Ames test. | [56] |
| Triploid onium Allium cornutum Clementi ex Visiani, 1842, and common onion Allium cepa L. | Proliferation assay by MTS DNA fragmentation assay PCR of p53, Bax, Caspase 3 | Hela, HCT116, and U2OS human cancer cell lines | Serial dilutions of extracts from both Allium species (containing sulfides) were added to the 3 cell lines. | Antiproliferative effects of both species were reported in the three cell lines. They induced apoptosis in HeLa cells. | [94] |
| Allicin | Determination of LC50 DNA fragmentation assay | Schistosoma mansoni | Not revealed | LC50 = 315 µL/L No DNA fragmentation | [95] |
3.4. Risk of Bias
| Reference | Clear Objective | Well Characterized Product | Reproducibility of the Assay | Comparability | Adequate Statistical Analysis | Total | Risk of Bias | General Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| [57] | 2 | 2 | 1 | 2 | 0 | 7 | 3 | M |
| [58] | 2 | 2 | 1 | 2 | 0 | 7 | 3 | M |
| [59] | 1 | 2 | 1 | 1 | 2 | 7 | 3 | M |
| [60] | 2 | 2 | 2 | 1 | 1 | 8 | 2 | L |
| [61] | 2 | 1 | 1 | 2 | 2 | 8 | 2 | L |
| [62] | 2 | 2 | 1 | 1 | 2 | 8 | 2 | L |
| [63] | 2 | 0 | 2 | 2 | 2 | 8 | 2 | L |
| [64] | 2 | 2 | 2 | 2 | 2 | 10 | 0 | L |
| [65] | 2 | 1 | 2 | 2 | 2 | 9 | 1 | L |
| [66] | 2 | 2 | 2 | 1 | 0 | 7 | 3 | M |
| [67] | 2 | 2 | 1 | 1 | 2 | 8 | 2 | L |
| [68] | 2 | 2 | 2 | 2 | 2 | 10 | 0 | L |
| [69] | 1 | 1 | 2 | 2 | 0 | 6 | 4 | M |
| [70] | 2 | 2 | 1 | 2 | 0 | 5 | 5 | M |
| [71] | 2 | 0 | 2 | 2 | 2 | 8 | 2 | L |
| [72] | 2 | 2 | 1 | 2 | 2 | 9 | 1 | L |
| [75] | 1 | 2 | 1 | 2 | 2 | 8 | 2 | L |
| [73] | 2 | 2 | 2 | 2 | 2 | 10 | 0 | L |
| [74] | 2 | 2 | 1 | 2 | 2 | 9 | 1 | L |
| [76] | 2 | 1 | 1 | 2 | 0 | 6 | 4 | M |
| [77] | 2 | 0 | 2 | 2 | 2 | 8 | 2 | L |
| [78] | 2 | 2 | 2 | 2 | 1 | 9 | 1 | L |
| [79] | 2 | 2 | 0 | 2 | 2 | 8 | 2 | L |
| [80] | 2 | 2 | 1 | 2 | 2 | 9 | 1 | L |
| [81] | 2 | 2 | 2 | 2 | 0 | 8 | 2 | L |
| [82] | 2 | 0 | 1 | 2 | 2 | 7 | 3 | M |
| [83] | 2 | 2 | 1 | 1 | 2 | 8 | 2 | L |
| [84] | 1 | 2 | 1 | 1 | 2 | 7 | 3 | L |
| [85] | 2 | 2 | 2 | 1 | 2 | 9 | 1 | L |
| [16] | 2 | 2 | 2 | 2 | 2 | 10 | 0 | L |
| [17] | 2 | 2 | 2 | 2 | 2 | 10 | 0 | L |
| [86] | 2 | 2 | 1 | 1 | 2 | 8 | 2 | L |
| [87] | 2 | 2 | 1 | 2 | 2 | 9 | 1 | L |
| [88] | 2 | 2 | 2 | 2 | 2 | 10 | 0 | L |
| [89] | 2 | 2 | 2 | 2 | 0 | 8 | 2 | L |
| [55] | 2 | 2 | 2 | 2 | 2 | 10 | 0 | L |
| [91] | 2 | 2 | 2 | 1 | 2 | 9 | 1 | L |
| [92] | 2 | 1 | 1 | 1 | 2 | 8 | 2 | L |
| [90] | 2 | 2 | 2 | 2 | 2 | 10 | 0 | L |
| [93] | 2 | 1 | 1 | 2 | 0 | 6 | 4 | M |
| [56] | 2 | 0 | 2 | 1 | 2 | 7 | 3 | M |
| [94] | 2 | 2 | 2 | 2 | 2 | 10 | 0 | L |
| [95] | 2 | 2 | 0 | 0 | 0 | 4 | 6 | H |
3.5. Limitations
3.6. In Vivo Studies Excluded
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Poojary, M.M.; Putnik, P.; Bursać Kovačević, D.; Barba, F.J.; Lorenzo, J.M.; Dias, D.A.; Shpigelman, A. Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. J. Food Compos. Anal. 2017, 61, 28–39. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Bursać Kovačević, D. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.A.; Locatelli, D.A.; González, R.E.; Cavagnaro, P.F.; Camargo, A.B. Analytical methods for bioactive sulfur compounds in Allium: An integrated review and future directions. J. Food Compos. Anal. 2017, 61, 4–19. [Google Scholar] [CrossRef]
- Ariza, J.J.; García-López, D.; Sánchez-Nieto, E.; Guillamón, E.; Baños, A.; Martínez-Bueno, M. Antilisterial effect of a natural formulation based on citrus extract in ready-to-eat foods. Foods 2021, 10, 1475. [Google Scholar] [CrossRef]
- Deka, B.; Manna, P.; Borah, J.C.; Talukdar, N.C. A review on phytochemical, pharmacological attributes and therapeutic uses of Allium hookeri. Phytomed. Plus 2022, 2, 100262. [Google Scholar] [CrossRef]
- Guillamón, E.; Andreo-Martínez, P.; Mut-Salud, N.; Fonollá, J.; Baños, A. Beneficial effects of organosulfur compounds from Allium cepa on gut health: A systematic review. Foods 2021, 10, 1680. [Google Scholar] [CrossRef] [PubMed]
- Rabelo-Ruiz, M.; Teso-Pérez, C.; Peralta-Sánchez, J.M.; Ariza, J.J.; Martín-Platero, A.M.; Casabuena-Rincón, Ó.; Vázquez-Chas, P.; Guillamón, E.; Aguinaga-Casañas, M.A.; Maqueda, M.; et al. Allium extract implements weaned piglet’s productive parameters by modulating distal gut microbiota. Antibiotics 2021, 10, 269. [Google Scholar] [CrossRef]
- Rabelo-Ruiz, M.; Ariza-Romero, J.J.; Zurita-González, M.J.; Martín-Platero, A.M.; Baños, A.; Maqueda, M.; Valdivia, E.; Martínez-Bueno, M.; Peralta-Sánchez, J.M. Allium-based phytobiotic enhances egg production in laying hens through microbial composition changes in ileum and cecum. Animals 2021, 11, 448. [Google Scholar] [CrossRef]
- Sharma, P.; Hajam, Y.A.; Kumar, R.; Rai, S. Complementary and alternative medicine for the treatment of diabetes and associated complications: A review on therapeutic role of polyphenols. Phytomed. Plus 2022, 2, 100188. [Google Scholar] [CrossRef]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Ariza-Romero, J.J.; Baños-Arjona, A.; Exposito-Ruiz, M.; Gutierrez-Fernandez, J. In vitro antibacterial activity of propyl-propane-thiosulfinate and propyl-propane-thiosulfonate derived from Allium spp. against gram-negative and gram-positive multidrug-resistant bacteria isolated from human samples. Biomed Res. Int. 2018, 2018, 7861207. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Lee, K.E.; Ambati, R.R.; Gundamaraju, R.; Ramadan, M.F.; Kang, S.G. Recent development in black garlic: Nutraceutical applications and health-promoting phytoconstituents. Food Rev. Int. 2021, 1–22. [Google Scholar] [CrossRef]
- Rochetti, G.; Chang, L.; Bocchi, S.; Giuberti, G.; Ak, G.; Elbasan, F.; Yildiztugay, E.; Ceylam, R.; Caree Nancy Picot-Allain, M.; Fawzi Mahomoodally, M.; et al. The functional potential of nine Allium species related to their untargeted phytochemical characterization, antioxidant capacity and enzyme inhibitory ability. Food Chem. 2022, 368, 130782. [Google Scholar] [CrossRef] [PubMed]
- Teshika, J.D.; Zakariyyah, A.M.; Zaynab, T.; Zengin, G.; Rengasamy, K.R.; Pandian, S.K.; Fawzi, M.M. Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, S39–S70. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Andrade, M.; Melo, N.R.; de Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Maisanaba, S.; Gutiérrez-Praena, D.; Prieto, A.I.; Pichardo, S.; Jos, Á.; Moreno, F.J.; Cameán, A.M. Cytotoxic and mutagenic in vitro assessment of two organosulfur compounds derived from onion to be used in the food industry. Food Chem. 2015, 166, 423–431. [Google Scholar] [CrossRef]
- Mellado-García, P.; Maisanaba, S.; Puerto, M.; Llana-Ruiz-Cabello, M.; Prieto, A.I.; Marcos, R.; Pichardo, S.; Cameán, A.M. Genotoxicity assessment of propyl thiosulfinate oxide, an organosulfur compound from Allium extract, intended to food active packaging. Food Chem. Toxicol. 2015, 86, 365–373. [Google Scholar] [CrossRef]
- Mylona, K.; Garcia-Cela, E.; Sulyok, M.; Medina, A.; Naresh, M. Influence of Two Garlic-Derived Compounds, Propyl Propane Thiosulfonate (PTS) and Propyl Propane Thiosulfinate (PTSO), on Growth and Mycotoxin Production by Fusarium Species In Vitro and in Stored Cereals. Toxins 2019, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Llana-Ruíz-Cabello, M.; Pichardo, S.; Jiménez-Morillo, N.T.; Abad, P.; Guillamón, E.; González-Vila, F.J.; Cameán, A.M.; González-Pérez, J.A. Characterisation of a bio-based packaging containing a natural additive from Allium spp. using analytical pyrolysis and carbon stable isotopes. J. Anal. Appl. Pyrolysis. 2016, 120, 334–340. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Moreno, F.J.; Baños, A.; Nuñez, C.; Guillamón, E.; Cameán, A.M. Acute toxicological studies of the main organosulfur compound derived from Allium sp. intended to be used in active food packaging. Food Chem. Toxicol. 2015, 82, 1–11. [Google Scholar] [CrossRef]
- Yoshida, H.; Iwata, N.; Katsuzaki, H.; Naganawa, R.; Ishikawa, K.; Fukuda, H.; Fujino, T.; Suzuki, A. Antimicrobial activity of a compound isolated from an oil-macerated garlic extract. Biosci. Biotechnol. Biochem. 1998, 62, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Ryu, J.; Surh, Y. Ajoene, a Major Organosulfide Found in Crushed Garlic, Induces NAD(P)H:quinone Oxidoreductase Expression Through Nuclear Factor E2-related Factor-2 Activation in Human Breast Epithelial Cells. J. Cancer Prev. 2019, 2, 112. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.Y. The antioxidant properties of garlic compounds: Alyl cysteine, alliin, allicin, and allyl disulfide. J. Med. Food. 2006, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, G.; Jamil, A.; Naor, R.; Tal, G.; Ludmer, Z.; Steinberg, D. Garlic allicin as a potential agent for controlling oral pathogens. J. Med. Food. 2014, 14, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, E.A.; Hill, D.J.; Maslin, D.J. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Env. Microbiol. 2000, 66, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.W.; Campopiano, D.J. Garlic revisited: Antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacia complex. PLoS ONE 2014, 9, e112726. [Google Scholar] [CrossRef]
- Yamada, Y.; Azuma, K. Evaluation of the in vitro antifungal activity of allicin. Antimicrob. Agents Chemother. 1977, 11, 743–749. [Google Scholar] [CrossRef]
- Gong-chen, W.; Lu-lu, H.; Jing, W.; Wan-nan, L.; Chuan-yi, P.; Yan-fei, L. Effects of Allicin on Lipid Metabolism and Antioxidant Activity in Chickens. J. Northeast. Agric. Univ. 2014, 21, 46–49. [Google Scholar] [CrossRef]
- Li, F.; Li, Q.; Wu, S.; Tan, Z. Salting-out extraction of allicin from garlic (Allium sativum L.) based on ethanol/ammonium sulfate in laboratory and pilot scale. Food Chem. 2017, 217, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.M.; Yin, M.C. In-vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oils. J. Med. Microbiol. 2001, 50, 646–649. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Luo, Y.; Nian, Y.; Liu, D.; Yin, X.; Wu, J.; Di, J.; Zhang, R.; Zhang, J. Diallyl Disulfide Suppresses the Inflammation and Apoptosis Resistance Induced by DCA through ROS and the NF-κB Signaling Pathway in Human Barrett’s Epithelial Cells. Inflammation 2017, 40, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Mathan Kumar, M.; Tamizhselvi, R. Protective effect of diallyl disulfide against cerulein-induced acute pancreatitis and associated lung injury in mice. Int. Immunopharmacol. 2000, 80, 106136. [Google Scholar] [CrossRef]
- Kalayarasan, S.; Prabhu, P.N.; Sriram, N.; Manikandan, R.; Arumugam, M.; Sudhandiran, G. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur. J. Pharmacol. 2009, 606, 162–171. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Wang, C.C.; Lai, T.Y.; Tsu, H.N.; Wang, C.H.; Liang, H.Y.; Kuo, W.W. Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int. J. Cardiol. 2013, 168, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Peinado, M.J.; Ruiz, R.; Echávarri, A.; Aranda-Olmedo, I.; Rubio, L.A. Garlic derivative PTS-O modulates intestinal microbiota composition and improves digestibility in growing broiler chickens. Anim. Feed Sci. Technol. 2013, 181, 87–92. [Google Scholar] [CrossRef]
- Peinado, M.J.; Ruiz, R.; Echávarri, A.; Rubio, L.A. Garlic derivative propyl propane thiosulfonate is effective against broiler enteropathogens in vivo. Poult. Sci. 2012, 91, 2148–2157. [Google Scholar] [CrossRef]
- Falcón-Piñeiro, A.; Remesal, E.; Noguera, M.; Ariza, J.J.; Guillamón, E.; Baños, A.; Navas-Cortes, J.A. Antifungal activity of propyl-propane-thiosulfinate (PTS) and propyl-propane-thiosulfonate (PTSO) from Allium cepa against Verticillium dahliae: In vitro and in planta assays. J. Fungi 2021, 7, 736. [Google Scholar] [CrossRef] [PubMed]
- Bravo, D.; Lillehoj, H. Use of At Least One Dialkyl Thosulfonate or Thosulfinate for Reducing the Number of Apcomplexan an Animal. U.S. Patent 2013/0079402 A1, 28 March 2013. Available online: https://patentimages.storage.googleapis.com/5b/df/ca/82319693e99358/US20130079402A1.pdf (accessed on 19 July 2022).
- Nuñez Lechado, C.; Baños Arjona, A.; Guillamon Ayala, E.; Valero López, A.; Navarro Moll, M.C.; Sanz, A. Use of Propyl Propanethosulfinate and Propyl Propanethosulfonate for the Prevention and Reduction of Parasites in Aquatic Animals. U.S. Patent 9,271,947 B2, 1 March 2016. Available online: https://patentimages.storage.googleapis.com/db/7d/6d/a1e24b67f01c40/US9271947.pdf (accessed on 19 July 2022).
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Gil-Martinez, L.; Ariza-Romero, J.J.; Maroto-Tello, A.; Baños-Arjona, A.; Gutierrez-Fernandez, J. Antibacterial and antifungal activity of propyl-propane-thiosulfinate and propyl-propane-thiosulfonate, two organosulfur compounds from Allium cepa: In vitro antimicrobial effect via the gas phase. Pharmaceuticals 2021, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, P.D.; Barrera, D.; Rivero, I.; Mata, R.; Medina-Campos, O.N.; Hernández-Pando, R.; Pedraza-Chaverrí, J. Antioxidant S-allylcysteine prevents gentamicin-induced oxidative stress and renal damage. Free Radic. Biol. Med. 2003, 35, 317–324. [Google Scholar] [CrossRef]
- Higuchi, O.; Tateshita, K.; Nishimura, H. Antioxidative Activity of Sulfur-Containing Compounds in Allium Species for Human Low-Density Lipoprotein (LDL) Oxidation in vitro. J. Agric. Food Chem. 2003, 51, 7208–7214. [Google Scholar] [CrossRef]
- Kahl, R.; Kappus, H. Toxikologie der synthetischen Antioxidantien BHA und BHT im Vergleich mit dem natürlichen Antioxidans Vitamin E. Z. Lebensm. Unters. Forsch. 1993, 196, 329–338. [Google Scholar] [CrossRef]
- Baños, A.; García, J.D.; Núñez, C.; Mut-Salud, N.; Ananou, S.; Martínez-Bueno, M.; Maqueda, M.; Valdivia, E. Subchronic toxicity study in BALBc mice of enterocin AS-48, an anti-microbial peptide produced by Enterococcus faecalis UGRA10. Food Chem. Toxicol. 2019, 132, 110667. [Google Scholar] [CrossRef] [PubMed]
- Maisanaba, S.; Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Puerto, M.; Prieto, A.I.; Jos, A.; Cameán, A.M. New advances in active packaging incorporated with essential oils or their main components for food preservation. Food Rev. Int. 2017, 33, 447–515. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bermudez, J.M.; Baños, A.; Ariza, J.J.; Guillamón, E.; Aucejo, S.; Cameán, A.M. Characterisation and antimicrobial activity of active polypropylene films containing oregano essential oil and Allium extract to be used in packaging for meat products. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Guidance for submission for food additive evaluations. EFSA J. 2012, 10, 2760. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2760 (accessed on 3 January 2022).
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific opinion on recent developments in the risk assessment of chemicals in food and their potential impact on the safety assessment of sub-stances used in food contact materials. EFSA J. 2016, 14, 4357. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4357 (accessed on 3 January 2022).
- EFSA FEEDAP Panel (EFSA Panel on Products or Substances used in Animal Feed). Guidance on the assessment of the safety of feed additives for the consumer. EFSA J. 2017, 15, e05022. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/5022 (accessed on 3 January 2022).
- EFSA Scientific Committee. Scientific Opinion on the clarification of some aspects related to genotoxicity assessment. EFSA J. 2017, 15, 5113. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/5113 (accessed on 3 January 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 89. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Viswanathan, M.; Patnode, C.D.; Berkman, N.D.; Bass, E.B.; Chang, S.; Hartling, L.; Murad, M.H.; Treadwell, J.R.; Kane, R.L. Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. J. Clin. Epidemiol. 2018, 97, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Mellado-García, P.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Marcos, R.; Pichardo, S.; Cameán, A.M. In vitro toxicological assessment of an organosulfur compound from Allium extract: Cytotoxicity, mutagenicity and genotoxicity studies. Food Chem. Toxicol. 2017, 99, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Barreto, M.; Aleixo, N.A.; Silvestre, R.B.; Fregonezi, N.F.; Barud, H.D.S.; Dias, D.D.S.; Ribeiro, C.A.; Resende, F.A. Genotoxicological safety assessment of puree-only edible films from onion bulb (Allium cepa L.) for use in food packaging-related applications. J. Food Sci. 2019, 85, 201–208. [Google Scholar] [CrossRef]
- Scharfenberg, K.; Wagner, R.; Wagner, K.G. The cytotoxic effect of ajoene, a natural product from garlic, investigated with different cell lines. Cancer Lett. 1990, 53, 103–108. [Google Scholar] [CrossRef]
- Scharfenberg, K.; Ryll, T.; Wagner, R.; Wagner, K.G. Injuries to cultivated BJA-B cells by ajoene, a garlic-derived natural compound: Cell viability, glutathione metabolism, and pools of acidic amino acids. J. Cell. Physiol. 1994, 158, 55–60. [Google Scholar] [CrossRef]
- Musk, S.R.R.; Smith, T.K.; Stening, P.; Fyfe, D.; Johnson, I.T.; Stephenson, P. Selective Toxicity of Compounds Naturally Present in Food Toward the Transformed Phenotype of Human Colorectal Cell Line HT29. Nutr. Cancer 1990, 24, 289–298. [Google Scholar] [CrossRef]
- Musk, S.R.R.; Clapham, P.; Johnson, I.T. Cytotoxicity and Genotoxicity of Diallyl Sulfide and Diallyl Disulfide Towards Chinese Hamster Ovary Cells. Food Chem. Toxicol. 1997, 35, 379–385. [Google Scholar] [CrossRef]
- Sigounas, G.; Hooker, J.L.; Li, W.; Anagnostou, A.; Steiner, M. S-allylmercaptocysteine, a stable thioallyl compound, induces apoptosis in erythroleukemia cell lines. Nutr. Cancer 1997, 28, 153–159. [Google Scholar] [CrossRef]
- Guyonnet, D.; Belloir, C.; Suschetet, M.; Siess, M.H.; Le Bon, A.M. Liver subcellular fractions from rats treated by organosulfur compounds from Allium modulate mutagen activation. Mutat. Res.-Genet. Toxicol. Env. Mutagen. 2000, 466, 17–26. [Google Scholar] [CrossRef]
- Hong, Y.S.; Ham, Y.A.; Choi, J.H.; Kim, J. Effects of allyl sulfur compounds and garlic extract on the expressions of Bcl-2, Bax, and p53 in non small cell lung cancer cell lines. Exp. Mol. Med. 2000, 32, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; Aquilano, K.; Rotilio, G.; Ciriolo, M.R. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003, 63, 5940–5949. [Google Scholar] [PubMed]
- Kim, J.G.; Koh, S.H.; Lee, Y.J.; Lee, K.Y.; Kim, Y.; Kim, S.; Lee, M.K.; Kim, S.H. Differential effects of diallyl disulfide on neuronal cells depend on its concentration. Toxicology 2005, 211, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Cho, S.J.; Kwon, H.; Lee, K.R.; Rhee, D.K.; Pyo, S. Corrigendum to “Caspase-independent cell death by allicin in human epithelial carcinoma cells: Involvement of PKA”. Cancer Lett. 2005, 224, 123–132. [Google Scholar] [CrossRef]
- Taylor, P.; Noriega, R.; Farah, C.; Abad, M.J.; Arsenak, M.; Apitz, R. Ajoene inhibits both primary tumor growth and metastasis of B16/BL6 melanoma cells in C57BL/6 mice. Cancer Lett. 2006, 239, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Banik, N.L.; Ray, S.K. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer 2007, 110, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Prager-Khoutorsky, M.; Goncharov, I.; Rabinkov, A.; Mirelman, D.; Geiger, B.; Bershadsky, A.D. Allicin inhibits cell polarization, migration and division via its direct effect on microtubules. Cell Motil. Cytoskeleton. 2007, 64, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.Y.; Yao, S.Q.; Zu, X.Y.; Huang, Z.X.; Liu, L.J.; Zhong, M.; Zhu, B.Y.; Tang, S.S.; Liao, D.F. Apoptosis induced by diallyl disulfide in human breast cancer cell line MCF-7. Acta Pharmacol. Sin. 2008, 29, 1233–1239. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Ashokkumar, P.; Sureshkumar, A.; Sudhandiran, G. Diallyl sulfide induces apoptosis in Colo 320 DM human colon cancer cells: Involvement of caspase-3, NF-κB, and ERK-2. Mol. Cell. Biochem. 2008, 311, 157–165. [Google Scholar] [CrossRef]
- Tan, H.; Ling, H.; He, J.; Yi, L.; Zhou, J.; Lin, M.; Su, Q. Inhibition of ERK and activation of p38 are involved in diallyl disulfide induced apoptosis of leukemia HL-60 cells. Arch. Pharm. Res. 2008, 31, 786–793. [Google Scholar] [CrossRef]
- Ledezma, E.; Wittig, O.; Alonso, J.; Cardier, J.E. Potentiated cytotoxic effects of statins and ajoene in murine melanoma cells. Melanoma Res. 2009, 19, 69–74. [Google Scholar] [CrossRef]
- Zhou, C.; Mao, X.P.; Guo, Q.; Zeng, F.Q. Diallyl trisulphide-induced apoptosis in human melanoma cells involves downregulation of Bcl-2 and Bcl-xL expression and activation of caspases. Clin. Exp. Dermatol. 2009, 34, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Jun, Z.; Suzuki, M.; Xiao, J.; Wen, J.; Talbot, S.G.; Li, G.C.; Xu, M. Comparative effects of natural and synthetic diallyl disulfide on apoptosis of human breast-cancer MCF-7 cells. Biotechnol. Appl. Biochem. 2009, 52, 113. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Park, B.-H.; Kim, S.-Y.; Lee, Y.J. Role of Bim in diallyl trisulfide-induced cytotoxicity in human cancer cells. J. Cell. Biochem. 2011, 112, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.P.; Chung, H.W.; Liu, K.C.; Wu, R.S.C.; Yang, J.S.; Tang, N.Y.; Lo, C.; Hsia, T.E.C.; Yu, C.C.; Chueh, F.U.S.; et al. Diallyl sulfide induces cell cycle arrest and apoptosis in HeLa human cervical cancer cells through the p53, caspase- and mitochondria-dependent pathways. Int. J. Oncol. 2011, 38, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, A.; Sielicka-Dudzin, A.; Herman-Antosiewicz, A.; Wozniak, M.; Fedeli, D.; Falcioni, G.; Antosiewicz, J. Diallyl trisulfide-induced prostate cancer cell death is associated with Akt/PKB dephosphorylation mediated by P-p66shc. Eur. J. Nutr. 2012, 51, 817–825. [Google Scholar] [CrossRef]
- Sielicka-Dudzin, A.; Borkowska, A.; Herman-Antosiewicz, A.; Wozniak, M.; Jozwik, A.; Fedeli, D.; Antosiewicz, J. Impact of JNK1, JNK2, and ligase Itch on reactive oxygen species formation and survival of prostate cancer cells treated with diallyl trisulfide. Eur. J. Nutr. 2012, 51, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.S.; Huang, A.C.; Lai, K.C.; Huang, Y.P.; Lin, M.W.; Yang, J.S.; Chung, J.G. Diallyl trisulfide induces apoptosis in human primary colorectal cancer cells. Oncol. Rep. 2012, 28, 949–954. [Google Scholar] [CrossRef]
- Borkowska, A.; Knap, N.; Antosiewicz, J. Diallyl trisulfide is more cytotoxic to prostate cancer cells PC-3 than to noncancerous epithelial cell line PNT1A: A possible role of p66Shc signaling axis. Nutr. Cancer 2013, 65, 711–717. [Google Scholar] [CrossRef]
- Tepe, B.; Tuncer, E.; Saraydän, S.U.; Özer, H.; Şen, M.; Karadayi, K.; Inan, D.S.; Karadayi, S.; Polat, Z.; Akpulat, A.; et al. Antitumoral effects of Allium sivasicum on breast cancer in vitro and in vivo. Mol. Biol. Rep. 2013, 40, 597–604. [Google Scholar] [CrossRef]
- Tao, M.; Gao, L.; Pan, J.; Wang, X. Study on the inhibitory effect of allicin on human gastric cancer cell line SGC-7901 and its mechanism. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, J.; Li, X.; Liu, D.; Feng, C.; Liang, R.; Zhuang, K.; Cai, C.; Xue, X.; Jing, F.; et al. DADS suppresses human esophageal xenograft tumors through RAF/MEK/ERK and mitochondria-dependent pathways. Int. J. Mol. Sci. 2014, 15, 12422–12441. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, R.; Feng, C.; Zhang, J.; Liu, D.; Xu, K.; Wang, X.; Zhang, S.; Li, Z.; Liu, X.; et al. Diallyl disulfide induces G2/M arrest and promotes apoptosis through the p53/p21 and MEK-ERK pathways in human esophageal squamous cell carcinoma. Oncol. Rep. 2014, 32, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Duan, W.; Feng, C.; He, X. Allicin induces apoptosis of the MGC-803 human gastric carcinoma cell line through the p38 mitogen-activated protein kinase/caspase-3 signaling pathway. Mol. Med. Rep. 2015, 11, 2755–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigemi, Z.; Furukawa, Y.; Hosokawa, K.; Minami, S.; Matsuhiro, J.; Nakata, S.; Watanabe, T.; Kagawa, H.; Nakagawa, K.; Takeda, H.; et al. Diallyl trisulfide induces apoptosis by suppressing NF-B signaling through destabilization of TRAF6 in primary effusion lymphoma. Int. J. Oncol. 2016, 48, 293–304. [Google Scholar] [CrossRef]
- Choi, Y.H. Diallyl trisulfide induces apoptosis and mitotic arrest in AGS human gastric carcinoma cells through reactive oxygen species-mediated activation of AMP-activated protein kinase. Biomed. Pharmacother. 2017, 94, 63–71. [Google Scholar] [CrossRef]
- Hwang, J.S.; Lee, Y.Y.; Lee, D.H.; Kwon, K.H. DATS sensitizes glioma cells to TRAIL-mediated apoptosis by up-regulation of death receptor 5 via ROS. Food Chem. Toxicol. 2017, 106, 514–521. [Google Scholar] [CrossRef]
- Li, C.; Jing, H.; Ma, G.; Liang, P. Allicin induces apoptosis through activation ofboth intrinsic and extrinsic pathways in glioma cells. Mol. Med. Rep. 2018, 17, 5976–5981. [Google Scholar] [CrossRef]
- Kema, V.H.; Khan, I.; Kapur, S.; Mandal, P. Evaluating the effect of diallyl sulfide on regulation of inflammatory mRNA expression in 3T3L1 adipocytes and RAW 264.7 macrophages during ethanol treatment. Drug Chem. Toxicol. 2018, 41, 302–313. [Google Scholar] [CrossRef]
- Jobani, B.M.; Najafzadeh, N.; Mazani, M.; Arzanlou, M.; Vardin, M.M. Molecular mechanism and cytotoxicity of allicin and all-trans retinoic acid against CD44+ versus CD117+ melanoma cells. Phytomedicine 2018, 48, 161–169. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, S.; Kim, D.Y.; You, S.; Park, D.; Oh, S.C.; Lee, D.H. Diallyl disulfide (DADS) boosts TRAIL-Mediated apoptosis in colorectal cancer cells by inhibiting Bcl-2. Food Chem. Toxicol. 2019, 125, 354–360. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Soldo, B.; Šprung, M.; Marijanović, Z.; Jerković, I.; Puizina, J. Comparison of organosulfur and amino acid composition between triploid onion Allium cornutum clementi ex visiani, 1842, and common onion Allium cepa L., and evidences for antiproliferative activity of their extracts. Plants 2020, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Rashed, H.A.E.H.; Abu Almaaty, A.H.; Soliman, M.F.M.; El-Shenawy, N.S. The in vitro antischistosomal activity and genotoxicity of the active ingredients of Allium sativum (Allicin) and Curcuma longa (curcumin). Iran. J. Parasitol. 2021, 16, 101–110. [Google Scholar] [CrossRef]
- Ferreira González, I.; Urrútia, G.; Alonso-Coello, P. Systematic Reviews and Meta-Analysis: Scientific Rationale and Interpretation. Rev. Esp. Cardiol. 2011, 64, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.G.; Milner, J.A. Diallyl disulfide suppresses the growth of human colon tumor cell xenografts in athymic nude mice. J. Nutr. 1996, 126, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Lee, D.T.W.; Tsao, S.W.; Wang, X.; Wong, Y.C. S-allylcysteine, a water-soluble garlic derivative, suppresses the growth of a human androgen-independent prostate cancer xenograft, CWR22R, under in vivo conditions. BJU Int. 2007, 9, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Yamada, N.; Hattori, A.; Fukuda, H.; Fujino, T. Inhibition by ajoene of skin-tumor promotion in mice. Biosci. Biotechnol. Biochem. 2022, 66, 2221–2223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, E.; Chen, F.; Yana, H.; Yuan, Y. Potential protective effects of oral administration of allicin on acrylamide-induced toxicity in male mice. Food Funct. 2013, 4, 1229–1236. [Google Scholar] [CrossRef]
- Cascajosa Lira, A.; Prieto, A.I.; Baños, A.; Guillamón, E.; Moyano, R.; Jos, Á.; Cameán, A.M. Safety assessment of Propil-propane-thiosulfonate (PTSO): 90-days oral subchronic toxicity study in rats. Food Chem. Toxicol. 2020, 144, 111612. [Google Scholar] [CrossRef]
- Mellado-Garcia, P.; Puerto, M.; Pichardo, S.; Llana-Ruiz-Cabello, M.; Moyano, R.; Blanco, A.; Jos, A.; Camean, A.M. Toxicological evaluation of an Allium-based commercial product in a 90-day feeding study in Sprague-Dawley rats. Food Chem. Toxicol. 2016, 90, 18–29. [Google Scholar] [CrossRef]
- Cascajosa-Lira, A.; Puerto, M.; Prieto, A.I.; Pichardo, S.; Díez-Quijada Jiménez, L.; Baños, A.; Guillamón, E.; Moyano, R.; Molina-Hernández, V.; Jos, Á.; et al. Genotoxicity Evaluation of Propyl-Propane-Thiosulfinate (PTS) from Allium genus Essential Oils by a Combination of Micronucleus and Comet Assays in Rats. Foods 2021, 10, 989. [Google Scholar] [CrossRef]
- Mellado-García, P.; Puerto, M.; Prieto, A.I.; Pichardo, S.; Martín-Cameán, A.; Moyano, R.; Blanco, A.; Cameán, A.M. Genotoxicity of a thiosulfonate compound derived from Allium sp. Intended to be used in active food packaging: In vivo comet assay and micronucleus test. Mutat Res Genet. Toxicol Env. Mutagen 2016, 800–801, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Dahiya, A.; Prakash, A.; Agrawala, P.K. Acute toxicity of diallyl sulfide derived from Allium sativum (garlic) in mice and its possible mechanisms. Phytomed. Plus 2021, 1, 100084. [Google Scholar] [CrossRef]


| Name | Chemical Structure | Mode of Action | Reference |
|---|---|---|---|
| E-Ajoene Z-Ajoene |  | Antibacterial: in vitro activity against Gram +: MIC = 5–250 µg/mL and Gram –: MIC = 100–500 µg/mL | [21] |
| Antifungal: MIC = 15–50 µg/mL | |||
| Antioxidant enzyme induction: NAD(P)H: quinone oxidoreductase-1 (NQO1) | [22] | ||
| Alliin |  | Antioxidant: superoxide scavenging activity | [23] |
| Allicin |  | Antibacterial: in vitro activity against Staphylococcus strains MIC = 600 µg/mL and Gram-bacteria: MIC = 4–300 µg/mL | [24,25,26] |
| Antifungal: MIC = 1.52–6.25 µg/mL | [27] | ||
| Antioxidant activity in vivo: SOD and GSH-Px activities increased. Radical scavenging ability of hydroxyl radical increase with Allicin | [24,28,29] | ||
| Di-allyl-disulphide (DADS) |  | Antibacterial activity against S. aureus: MIC = 4 µg/mL and Helicobacter pylori MIC = 200 µg/mL | [25,30] |
| Antifungal activity against Aspergillus spp: MIC = 8–12 µg/mL and Candida spp: MIC = 4–12 µg/mL | [30] | ||
| Antioxidant in vivo activity | [31,32] | ||
| Di-allyl-sulphide (DAS) |  | Antibacterial activity against S. aureus: MIC = 20 µg/mL and H. pylory MIC = 4 µg/mL | [25,30] |
| Antifungal activity against Aspergillus spp: MIC= 40–64 µg/mL and Candida spp: MIC= 32–72 µg/mL | [30] | ||
| Antioxidant in vivo activity | [33] | ||
| Di-allyl-trisulphide (DATS) |  | Antibacterial activity against S. aureus: MIC = 2 µg/mL and H. pylory MIC = 25 µg/mL | [25,30] |
| Antifungal activity against Aspergillus spp: MIC = 2–8 µg/mL and Candida spp: MIC = 1–8 µg/mL | [30] | ||
| Antioxidant in vitro activity | [34] | ||
| Dipropyl disulphide (DPDS) |  | Antioxidant in vitro activity | [16] |
| Dipropyl sulphide (DPS) |  | Antioxidant in vitro activity | [16] |
| Propyl-propane-tiosulphonate (PTSO) |  | Antibacterial activity against Gram +: MCB = 0.5–10 µg/mL and Gram-: MCB = 1.25–10 µg/mL | [20,35,36] |
| Antifungal: MFC against Verticillium dahliae = 19.53–39.06 µg/mL MFC90 against Candida spp. = 64–128 µg/mL | [11,37] | ||
| Antiprotozoal and Antiparasitic: reduce the number of apicomplexa in monogastric animals. Reducing a plurality of aquatic parasites in aquatic animals. | [38,39] | ||
| Antioxidant | [20] | ||
| Propyl-propane-tiosulphinate (PTS) |  | Antibacterial activity against Gram -: MCB = 128–1024 µg/mL and Gram +: MCB = 128 µg/mL | [11] |
| Antifungal activity MFC against Verticillium dahliae = 78.13 µg/mL MFC90 against Candida spp = 128 µg/mL | [34,40] | ||
| Antiprotozoal and antiparasitic: reduce the number of Apicomplexa in monogastric animals. Reducing a plurality of aquatic parasites in aquatic animals. | [38,39] | ||
| S-allylcysteine (SAC) |  | Antioxidant activity by scavenging ROS | [41] |
| Vinyldithiin |  | Antioxidant activity | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascajosa-Lira, A.; Andreo-Martínez, P.; Prieto, A.I.; Baños, A.; Guillamón, E.; Jos, A.; Cameán, A.M. In Vitro Toxicity Studies of Bioactive Organosulfur Compounds from Allium spp. with Potential Application in the Agri-Food Industry: A Review. Foods 2022, 11, 2620. https://doi.org/10.3390/foods11172620
Cascajosa-Lira A, Andreo-Martínez P, Prieto AI, Baños A, Guillamón E, Jos A, Cameán AM. In Vitro Toxicity Studies of Bioactive Organosulfur Compounds from Allium spp. with Potential Application in the Agri-Food Industry: A Review. Foods. 2022; 11(17):2620. https://doi.org/10.3390/foods11172620
Chicago/Turabian StyleCascajosa-Lira, Antonio, Pedro Andreo-Martínez, Ana Isabel Prieto, Alberto Baños, Enrique Guillamón, Angeles Jos, and Ana M. Cameán. 2022. "In Vitro Toxicity Studies of Bioactive Organosulfur Compounds from Allium spp. with Potential Application in the Agri-Food Industry: A Review" Foods 11, no. 17: 2620. https://doi.org/10.3390/foods11172620
APA StyleCascajosa-Lira, A., Andreo-Martínez, P., Prieto, A. I., Baños, A., Guillamón, E., Jos, A., & Cameán, A. M. (2022). In Vitro Toxicity Studies of Bioactive Organosulfur Compounds from Allium spp. with Potential Application in the Agri-Food Industry: A Review. Foods, 11(17), 2620. https://doi.org/10.3390/foods11172620