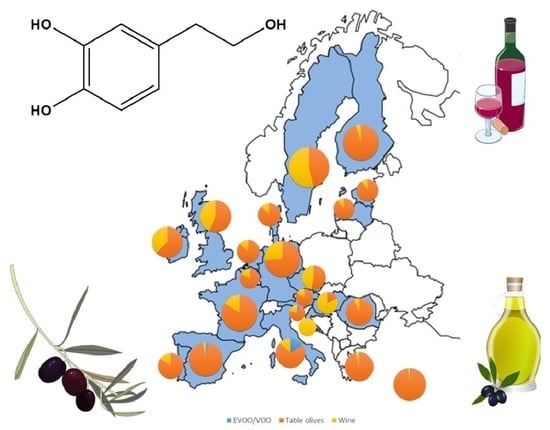
Hydroxytyrosol in Foods: Analysis, Food Sources, EU Dietary Intake, and Potential Uses
Abstract
:1. Introduction
2. Methodology
2.1. Literature Search
2.2. Estimation of Hydroxytyrosol Dietary Intake in Europe
3. HT Health Benefits and Mechanisms of Action
4. Determination of HT in Foods
5. Dietary Sources
5.1. Olives
5.2. Olive Oil
5.3. Wine
6. Estimation of HT Dietary Intake
7. Hydroxytyrosol as a Food Ingredient and Future Trends
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villaño, D.; Fernández-Pachón, M.S.; Troncoso, A.M.; García-Parrilla, M.C. Comparison of antioxidant activity of wine phenolic compounds and metabolites in vitro. Anal. Chim. Acta 2005, 538, 391–398. [Google Scholar] [CrossRef]
- Napolitano, A.; De Lucia, M.; Panzella, L.; d’Ischia, M. The chemistry of tyrosol and hydroxytyrosol: Implications for oxidative stress. Olives Olive Oil Health Dis. Prev. 2010, 134, 1225–1232. [Google Scholar] [CrossRef]
- Fitó, M.; Cladellas, M.; De La Torre, R.; Marti, J.; Alcántara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Bruguera, J.; López-Sabater, M.C.; Vila, J.; et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis 2005, 181, 149–158. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, N.; Herrera, M.; Frías, L.; Provencio, M.; Pérez-Carrión, R.; Díaz, V.; Morse, M.; Crespo, M.C. A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: Results of a pilot study. Clin. Transl. Oncol. 2019, 21, 489–498. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Alonso-Bellido, I.M.; Rodríguez-Gómez, J.A.; Troncoso, A.M.; García-Parrilla, M.C.; Venero, J.L.; Espinosa-Oliva, A.M.; De Pablos, R.M. Hydroxytyrosol decreases LPS-and α-synuclein-induced microglial activation in vitro. Antioxidants 2020, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Protective effects of hydroxytyrosol against α-synuclein toxicity on PC12 cells and fibril formation. Food Chem. Toxicol. 2018, 120, 41–49. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; García-Parrilla, M.C. Melatonin, protocatechuic acid and hydroxytyrosol effects on vitagenes system against alpha-synuclein toxicity. FCT 2019, 134, 110817. [Google Scholar] [CrossRef]
- St-Laurent-Thibault, C.; Arseneault, M.; Longpre, F.; Ramassamy, C. Tyrosol and hydroxytyrosol two main components of olive oil, protect N2a cells against amyloid-β-induced toxicity. involvement of the NF-κB signaling. Curr. Alzheimer Res. 2011, 8, 543–551. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Labrador, M.; Gutiérrez, A.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcia-Parrilla, M.C. Anti-VEGF signalling mechanism in HUVECs by melatonin, serotonin, hydroxytyrosol and other bioactive compounds. Nutrients 2019, 11, 2421. [Google Scholar] [CrossRef] [Green Version]
- Yehya, A.H.S.; Asif, M.; Petersen, S.H.; Subramaniam, A.V.; Kono, K.; Majid, A.M.S.A.; Oon, C.E. Angiogenesis: Managing the culprits behind tumorigenesis and metastasis. Medicina 2018, 54, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2002, 2, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Casaburi, I.; Rosano, C.; Avena, P.; De Luca, A.; Campana, C.; Martire, E.; Francesca Santolla, M.; Maggiolini, M.; Pezzi, V.; et al. Oleuropein and hydroxytyrosol activate GPER/GPR 30-dependent pathways leading to apoptosis of ER-negative SKBR 3 breast cancer cells. Mol. Nutr. Food Res. 2014, 58, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Talorete, T.P.; Yamada, P.; Isoda, H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database J. Biol. Databases Curation 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Moreno-González, R.; Juan, M.E.; Planas, J.M. Table olive polyphenols: A simultaneous determination by liquid chromatography–mass spectrometry. J. Chromatogr. A 2020, 1609, 460434. [Google Scholar] [CrossRef]
- Fernández, A.; Talaverano, M.I.; Pérez-Nevado, F.; Boselli, E.; Cordeiro, A.M.; Martillanes, S.; Foligni, R.; Martín-Vertedor, D. Evaluation of phenolics and acrylamide and their bioavailability in high hydrostatic pressure treated and fried table olives. J. Food Process. Preserv. 2020, 44, e14384. [Google Scholar] [CrossRef]
- Schaide, T.; Cabrera-Bañegil, M.; Pérez-Nevado, F.; Esperilla, A.; Martín-Vertedor, D. Effect of olive leaf extract combined with Saccharomyces cerevisiae in the fermentation process of table olives. J. Food Sci. Technol. 2019, 56, 3001–3013. [Google Scholar] [CrossRef]
- García, P.; Romero, C.; Brenes, M. Bioactive substances in black ripe olives produced in Spain and the USA. J. Food Compos. Anal. 2018, 66, 193–198. [Google Scholar] [CrossRef] [Green Version]
- García, P.; Romero, C.; Brenes, M. Influence of olive tree irrigation and the preservation system on the fruit characteristics of Hojiblanca black ripe olives. LWT Food Sci. Technol. 2014, 55, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Lodolini, E.M.; Cabrera-Bañegil, M.; Fernández, A.; Delgado-Adámez, J.; Ramírez, R.; Martín-Vertedor, D. Monitoring of acrylamide and phenolic compounds in table olive after high hydrostatic pressure and cooking treatments. Food Chem. 2019, 286, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Difonzo, G.; Calasso, M.; Cosmai, L.; De Angelis, M. Effects of olive leaf extract addition on fermentative and oxidative processes of table olives and their nutritional properties. Int. Food Res. J. 2019, 116, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Bruno, A.; Linsalata, V.; Minervini, F.; Garbetta, A.; Tufariello, M.; Mita, G.; Logrieco, A.F.; Bleve, G.; Cardinali, A. Fermented Apulian table olives: Effect of selected microbial starters on polyphenols composition, antioxidant activities and bioaccessibility. Food Chem. 2018, 248, 137–145. [Google Scholar] [CrossRef]
- Ambra, R.; Natella, F.; Bello, C.; Lucchetti, S.; Forte, V.; Pastore, G. Phenolics fate in table olives (Olea europaea L. cv. Nocellara del Belice) debittered using the Spanish and Castelvetrano methods. Int. Food Res. J. 2017, 100, 369–376. [Google Scholar] [CrossRef]
- Pereira, J.A.; Pereira, A.P.; Ferreira, I.C.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A. Table olives from Portugal: Phenolic compounds, antioxidant potential, and antimicrobial activity. J. Agric. Food Chem. 2006, 54, 8425–8431. [Google Scholar] [CrossRef]
- Johnson, R.; Melliou, E.; Zweigenbaum, J.; Mitchell, A.E. Quantitation of Oleuropein and Related Phenolics in Cured Spanish-Style Green, California-Style Black Ripe, and Greek-Style Natural Fermentation Olives. J. Agric. Food Chem. 2018, 66, 2121–2128. [Google Scholar] [CrossRef]
- Melliou, E.; Zweigenbaum, J.A.; Mitchell, A.E. Ultrahigh-pressure liquid chromatography triple-quadrupole tandem mass spectrometry quantitation of polyphenols and secoiridoids in California-style black ripe olives and dry salt-cured olives. J. Agric. Food Chem. 2015, 63, 2400–2405. [Google Scholar] [CrossRef]
- Mettouchi, S.; Sacchi, R.; Moussa, Z.O.; Paduano, A.; Savarese, M.; Tamendjari, A. Effect of Spanish style processing on the phenolic compounds and antioxidant activity of Algerian green table olives. Grasas y Aceites 2016, 67, e114. [Google Scholar] [CrossRef]
- Othman, N.B.; Roblain, D.; Chammen, N.; Thonart, P.; Hamdi, M. Antioxidant phenolic compounds loss during the fermentation of Chétoui olives. Food Chem. 2009, 116, 662–669. [Google Scholar] [CrossRef]
- Selli, S.; Kelebek, H.; Kesen, S.; Sonmezdag, A.S. GC-MS olfactometric and LC-DAD-ESI-MS/MS characterization of key odorants and phenolic compounds in black dry-salted olives. J. Sci. Food Agric. 2018, 98, 4104–4111. [Google Scholar] [CrossRef] [PubMed]
- Leporini, M.; Loizzo, M.R.; Tenuta, M.C.; Falco, T.; Sicari, V.; Pellicanò, T.M.; Tundis, R. Calabrian extra-virgin olive oil from Frantoio cultivar: Chemical composition and health properties. Emir. J. Food Agric. 2018, 9, 1014. [Google Scholar] [CrossRef]
- Deiana, P.; Santona, M.; Dettori, S.; Molinu, M.G.; Dore, A.; Culeddu, N.; Azara, E.; Naziri, E.; Tsimidou, M.Z. Can all the Sardinian varieties support the PDO “Sardegna” virgin olive oil? Eur. J. Lipid Sci. Technol. 2019, 121, 1800135. [Google Scholar] [CrossRef]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA health claim on olive oil polyphenols: Acid hydrolysis validation and total hydroxytyrosol and tyrosol determination in Italian virgin olive oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trombetta, D.; Smeriglio, A.; Marcoccia, D.; Giofrè, S.V.; Toscano, G.; Mazzotti, F.; Giovanazzi, A.; Lorenzetti, S. Analytical evaluation and antioxidant properties of some secondary metabolites in northern Italian mono-and multi-varietal extra virgin olive oils (EVOOs) from early and late harvested olives. Int. J. Mol. 2017, 18, 797. [Google Scholar] [CrossRef]
- Cioffi, G.; Pesca, M.S.; De Caprariis, P.; Braca, A.; Severino, L.; De Tommasi, N. Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chem. 2010, 121, 105–111. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Mulinacci, N.; Galardi, C.; Vincieri, F.F.; Liberatore, L.; Cichelli, A. HPLC and HRGC analyses of polyphenols and secoiridoid in olive oil. Chromatographia 2001, 53, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Franco, M.N.; Galeano-Díaz, T.; López, Ó.; Fernández-Bolaños, J.G.; Sánchez, J.; De Miguel, C.; Gil, M.V.; Martín-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298. [Google Scholar] [CrossRef]
- De Torres, A.; Espínola, F.; Moya, M.; Alcalá, S.; Vidal, A.M.; Castro, E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M. Analysis of total contents of hydroxytyrosol and tyrosol in olive oils. J. Agric. Food Chem. 2012, 60, 9017–9022. [Google Scholar] [CrossRef]
- García-Villalba, R.; Carrasco-Pancorbo, A.; Vázquez-Martín, A.; Oliveras-Ferraros, C.; Menéndez, J.A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. A 2-D-HPLC-CE platform coupled to ESI-TOF-MS to characterize the phenolic fraction in olive oil. Electrophoresis 2009, 30, 2688–2701. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Macià, A.; Romero, M.P.; Motilva, M.J. Improved liquid chromatography tandem mass spectrometry method for the determination of phenolic compounds in virgin olive oil. J. Chromatogr. A 2008, 1214, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rico, A.; Salvador, M.D.; La Greca, M.; Fregapane, G. Phenolic and volatile compounds of extra virgin olive oil (Olea europaea L. Cv. Cornicabra) with regard to fruit ripening and irrigation management. J. Agric. Food Chem. 2006, 54, 7130–7136. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Artemiou, A.; Giogios, I.; Kalogeropoulos, N. The impact of fruit maturation on bioactive microconstituents, inhibition of serum oxidation and inflammatory markers in stimulated PBMCs and sensory characteristics of Koroneiki virgin olive oils from Messenia, Greece. Food Funct. 2013, 4, 1185–1194. [Google Scholar] [CrossRef]
- Bajoub, A.; Medina-Rodríguez, S.; Gómez-Romero, M.; Bagur-González, M.G.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Assessing the varietal origin of extra-virgin olive oil using liquid chromatography fingerprints of phenolic compound, data fusion and chemometrics. Food Chem. 2017, 215, 245–255. [Google Scholar] [CrossRef]
- Douzane, M.; Tamendjari, A.; Abdi, A.K.; Daas, M.S.; Mehdid, F.; Bellal, M.M. Phenolic compounds in mono-cultivar extra virgin olive oils from Algeria. Grasas y Aceites 2013, 64, 285–294. [Google Scholar] [CrossRef]
- Ouni, Y.; Taamalli, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Zarrouk, M. Characterisation and quantification of phenolic compounds of extra-virgin olive oils according to their geographical origin by a rapid and resolutive LC–ESI-TOF MS method. Food Chem. 2011, 127, 1263–1267. [Google Scholar] [CrossRef]
- Kesen, S.; Kelebek, H.; Selli, S. Characterization of the volatile, phenolic and antioxidant properties of monovarietal olive oil obtained from cv. Halhali. J. Am. Oil Chem. Soc. 2013, 90, 1685–1696. [Google Scholar] [CrossRef]
- Dağdelen, A.; Tümen, G.; Ozcan, M.M.; Dündar, E. Phenolics profiles of olive fruits (Olea europaea L.) and oils from Ayvalık, Domat and Gemlik varieties at different ripening stages. Food Chem. 2013, 136, 41–45. [Google Scholar] [CrossRef]
- Wang, S.T.; Le, J.; Peng, R.; Li, Y. Efficient extraction and sensitive LC-MS quantification of hydroxytyrosol in wine, oil and plasma. Food Chem. 2020, 323, 126803, Advance online publication. [Google Scholar] [CrossRef]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Maffia, M. HPLC analysis of phenols in negroamaro and primitivo red wines from Salento. Foods 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žurga, P.; Vahčić, N.; Pasković, I.; Banović, M.; Staver, M.M. Croatian Wines from Native Grape Varieties Have Higher Distinct Phenolic (Nutraceutic) Profiles than Wines from Non-Native Varieties with the Same Geographic Origin. Chem. Biodivers. 2019, 16, e1900218. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fernández, M.A.; Fernández-Cruz, E.; Cantos-Villar, E.; Troncoso, A.M.; García-Parrilla, M.C. Determination of hydroxytyrosol produced by winemaking yeasts during alcoholic fermentation using a validated UHPLC–HRMS method. Food Chem. 2018, 242, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Boronat Rigol, A. Tyrosol and Its Endogenous Conversion into Hydroxytyrosol in Humans: Dietary Sources, Genetic Modulation and Clinical Effects. Ph.D. Thesis, Universitat Pompeu Fabra, Barcelona, Spain, 2020. Available online: https://hdl.handle.net/10803/668336 (accessed on 25 June 2022).
- Soldevila-Domenech, N.; Boronat, A.; Mateus, J.; Diaz-Pellicer, P.; Matilla, I.; Pérez-Otero, M.; Aldea-Perona, A.; de la Torre, R. Generation of the Antioxidant Hydroxytyrosol from Tyrosol Present in Beer and Red Wine in a Randomized Clinical Trial. Nutrients 2019, 11, 2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.; Rotilio, D.; Pastore, G.M.; Durán, N. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 2003, 82, 409–416. [Google Scholar] [CrossRef]
- Di Tommaso, D.; Calabrese, R.; Rotilio, D. Identification and quantitation of hydroxytyrosol in Italian wines. J. High Resolut. Chromatogr. 1998, 21, 549–553. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Cantos-Villar, E.; Palma, M.; Puertas, B. Direct liquid chromatography method for the simultaneous quantification of hydroxytyrosol and tyrosol in red wines. J. Agric. Food Chem. 2011, 59, 11683–11689. [Google Scholar] [CrossRef]
- EFSA. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA J. 2011, 9, 2097. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.H.; Luben, R.N.; Spencer, J.P.E.; Schroeter, H.; Khaw, K.T.; Kuhnle, G.G.C. Flavonoid intake in European adults (18 to 64 Years). PLoS ONE 2015, 10, 0128132. [Google Scholar] [CrossRef]
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009, 180, 421–432. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Ma, Y.; Wen, D. Hydroxytyrosol ameliorates insulin resistance by modulating endoplasmic reticulum stress and prevents hepatic steatosis in diet-induced obesity mice. J. Nutr. Biochem. 2018, 57, 180–188. [Google Scholar] [CrossRef] [PubMed]
- IOC. COI/T.20/Doc. No 29—Determination of Biophenols in Olive Oils by HPLC. In Standards, Methods and Guides; Decision DEC-III-10/106-VI/2017; IOC: Lausanne, Switzerland, 2017. [Google Scholar]
- Tasioula-Margari, M.; Tsabolatidou, E. Extraction, separation, and identification of phenolic compounds in virgin olive oil by HPLC-DAD and HPLC-MS. Antioxidants 2015, 4, 548–562. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Lozano-Sánchez, J.; Gámez, M.C.; Carretero, A.S.; Taamalli, A. Bioactive phenolic compounds from Olea europaea: A challenge for analytical chemistry. In Olive and Olive Oil Bioactive Constituents, 1st ed.; Boskou, D., Ed.; AOCS Press: Amsterdam, The Netherlands, 2015; pp. 261–298. [Google Scholar] [CrossRef]
- Schwarz, M.; Weber, F.; Durán-Guerrero, E.; Castro, R.; Rodríguez-Dodero, M.D.C.; García-Moreno, M.V.; Winterhalter, P.; Guillén-Sánchez, D. Hplc-dad-ms and antioxidant profile of fractions from amontillado sherry wine obtained using high-speed counter-current chromatography. Foods 2021, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Gallina-Toschi, T.; Fernández-Gutierrez, A. Analytical determination of polyphenols in olive oils. J. Sep. Sci. 2005, 28, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; García, A.; Ríos, P.; García, J.J.; Garrido, A. Use of 1-acetoxypinoresinol to authenticate Picual olive oils. Int. J. Food Sci. Technol. 2002, 37, 615–625. [Google Scholar] [CrossRef]
- Olmo-García, L.; Carrasco-Pancorbo, A. Chromatography-MS based metabolomics applied to the study of virgin olive oil bioactive compounds: Characterization studies, agro-technological investigations, and assessment of healthy properties. Trends Analyt. Chem. 2021, 135, 116153. [Google Scholar] [CrossRef]
- Boronat, A.; Soldevila-Domenech, N.; Rodríguez-Morató, J.; Martínez-Huélamo, M.; Lamuela-Raventós, R.M.; De la Torre, R. Beer phenolic composition of simple phenols, prenylated flavonoids and alkylresorcinols. Molecules 2020, 25, 2582. [Google Scholar] [CrossRef]
- Brenes, M.; De Castro, A. Transformation of oleuropein and its hydrolysis products during Spanish-style green olive processing. J. Sci. Food Agric. 1998, 77, 353–358. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Brenes, M.; Rejano, L.; García, P.; Sánchez, A.H.; Garrido, A. Biochemical Changes in Phenolic Compounds during Spanish-Style Green Olive Processing. J. Agric. Food Chem. 1995, 43, 2702–2706. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; García, P.; Garrido, A. Hydroxytyrosol 4-β-D-glucoside, an important phenolic compound in olive fruits and derived products. Food Res. Int. 2002, 50, 3835–3839. [Google Scholar] [CrossRef] [PubMed]
- Arroyo López, F.N.; Romero, C.; Durán Quintana, M.D.C.; López, A.L.; García, P.G.; Fernández, A.G. Kinetic study of the physicochemical and microbiological changes in “seasoned” olives during the shelf-life period. J. Agric. Food Chem. 2005, 53, 5285–5292. [Google Scholar] [CrossRef] [PubMed]
- Romero-Segura, C.; García-Rodríguez, R.; Sánchez-Ortiz, A.; Sanz, C.; Pérez, A.G. The role of olive β-glucosidase in shaping the phenolic profile of virgin olive oil. Food Res. Int. 2012, 45, 191–196. [Google Scholar] [CrossRef]
- Brenes, M.; Garcia, A.; Garcia, P.; Garrido, A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem. 2001, 49, 5609–5614. [Google Scholar] [CrossRef] [PubMed]
- Esposito Salsano, J.; Digiacomo, M.; Cuffaro, D.; Bertini, S.; Macchia, M. Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods 2022, 11, 1354. [Google Scholar] [CrossRef] [PubMed]
- Nissiotis, M.; Tasioula-Margari, M. Changes in antioxidant concentration of virgin olive oil during thermal oxidation. Food Chem. 2002, 77, 371–376. [Google Scholar] [CrossRef]
- Mas, A.; Guillamon, J.M.; Torija, M.J.; Beltran, G.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Bioactive compounds derived from the yeast metabolism of aromatic amino acids during alcoholic fermentation. BioMed. Res. Int. 2014, 2014, 898045. [Google Scholar] [CrossRef]
- Noh, H.; Freisling, H.; Assi, N.; Zamora-Ros, R.; Achaintre, D.; Affret, A.; Mancini, F.; Boutron-Ruault, M.C.; Flögles, A.; Boeing, H.; et al. Identification of urinary polyphenol metabolite patterns associated with polyphenol-rich food intake in adults from four European Countries. Nutrients 2017, 9, 796. [Google Scholar] [CrossRef]
- Schröder, H.; De La Torre, R.; Estruch, R.; Corella, D.; Martínez-González, M.A.; Salas-Salvadó, J.; Ros, E.; Arós, F.; Flores, G.; Civit, E.; et al. Alcohol consumption is associated with high concentrations of urinary hydroxytyrosol. Am. J. Clin. Nutr. 2009, 90, 1329–1335. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Mañá, C.; Farré, M.; Pujadas, M.; Mustata, C.; Menoyo, E.; Pastor, A.; Langohr, K.; De La Torre, R. Ethanol induces hydroxytyrosol formation in humans. Pharmacol. Res. 2015, 95–96, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Mañá, C.; Farré, M.; Rodríguez-Morató, J.; Papaseit, E.; Pujadas, M.; Fitó, M.; Robledo, P.; Covas, M.I.; Cheynier, V.; Meudec, E.; et al. Moderate consumption of wine, through both its phenolic compounds and alcohol content, promotes hydroxytyrosol endogenous generation in humans. A randomized controlled trial. Mol. Nutr. Food Res. 2015, 59, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boronat Rigol, A.; Mateus, J.; Soldevila-Domenech, N.; Guerra, M.; Rodríguez-Morató, J.; Varon, C.; Muñoz, D.; Barbosa, F.; Morales, J.C.; Gaedigk, A.; et al. Data on the endogenous conversion of tyrosol into hydroxytyrosol in humans. Data Br. 2019, 27, 104787. [Google Scholar] [CrossRef]
- De La Torre, R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 2008, 16, 245–247. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, 4728. [Google Scholar] [CrossRef]
- Attya, M.; Benabdelkamel, H.; Perri, E.; Russo, A.; Sindona, G. Effects of conventional heating on the stability of major olive oil phenolic compounds by tandem mass spectrometry and isotope dilution assay. Molecules 2010, 15, 8734–8746. [Google Scholar] [CrossRef] [PubMed]
- Ziauddeen, N.; Rosi, A.; Del Rio, D.; Amoutzopoulos, B.; Nicholson, S.; Page, P.; Scazzina, F.; Brighenti, F.; Sumantra, R.; Mena, P. Dietary intake of (poly)phenols in children and adults: Cross-sectional analysis of UK National Diet and Nutrition Survey Rolling Programme (2008–2014). Eur. J. Nutr. 2019, 58, 3183–3198. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Touillaud, M.; Kaaks, R.; Teucher, B.; Mattiello, A.; Grioni, S.; et al. Estimation of the intake of anthocyanidins and their food sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2011, 106, 1090–1099. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Implementing Decision (EU) 2017/2373 of 14 December 2017 authorising the placing on the market of hydroxytyrosol as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union 2017, L 337, 56–59. [Google Scholar]
- Ribeiro, T.B.; Bonifácio-Lopes, T.; Morais, P.; Miranda, A.; Nunes, J.; Vicente, A.A.; Pintado, M. Incorporation of olive pomace ingredients into yoghurts as a source of fibre and hydroxytyrosol: Antioxidant activity and stability throughout gastrointestinal digestion. J. Food Eng. 2021, 297, 110476. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Innovative natural functional ingredients from olive and citrus extracts in spanish-type dry-cured sausage “fuet”. Antioxidants 2021, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Martínez-López, S.; Baeza Arévalo, G.; Amigo-Benavent, M.; Sarriá, B.; Bravo-Clemente, L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016, 205, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Kranz, P.; Braun, N.; Schulze, N.; Kunz, B. Sensory quality of functional beverages: Bitterness perception and bitter masking of olive leaf extract fortified fruit smoothies. J. Food Sci. 2010, 75, S308–S311. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Martínez, L.; Castillo, J.; Ros, G. Hydroxytyrosol extracts, olive oil and walnuts as functional components in chicken sausages. J. Sci. Food Agric. 2017, 97, 3761–3771. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.; Occhipinti, P.S.; Romeo, F.V.; Timpanaro, N.; Musumeci, T.; Randazzo, C.L.; Caggia, C. Phenols recovered from olive mill wastewater as natural booster to fortify blood orange juice. Food Chem. 2022, 393, 133428. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Navarro, D.; Maunier, S.; Sigoillot, J.C.; Lorquin, J.; Delattre, M.; Simon, J.-L.; Asther, M.; Labat, M. Simple phenolic content in olive oil residues as a function of extraction systems. Food Chem. 2001, 75, 501–507. [Google Scholar] [CrossRef]
- Allouche, N.; Fki, I.; Sayadi, S. Toward a High Yield Recovery of Antioxidants and Purified Hydroxytyrosol from Olive Mill Wastewaters. J. Agric. Food Chem. 2004, 52, 267–273. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef]
- Bertin, L.; Ferri, F.; Scoma, A.; Marchetti, L.; Fava, F. Recovery of high added value natural polyphenols from actual olive mill wastewater through solid phase extraction. Chem. Eng. J. 2011, 171, 1287–1293. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Mozetič Vodopivec, B. Ultrasonic extraction of phenols from olive mill wastewater: Comparison with conventional methods. J. Agric. Food Chem. 2011, 59, 12725–12731. [Google Scholar] [CrossRef]
- Khoufi, S.; Hamza, M.; Sayadi, S. Enzymatic hydrolysis of olive wastewater for hydroxytyrosol enrichment. Bioresour. Technol. 2011, 102, 9050–9058. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, C.; Pellegrino, M.; Romano, E.; Claps, S.; Fallara, C.; Perri, E. Qualitative and Quantitative Analysis of Phenolic Compounds in Spray-Dried Olive Mill Wastewater. Front. Nutr. 2022, 8, 782693. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bolaños, J.; Rodríguez, G.; Gómez, E.; Guillén, R.; Jiménez, A.; Heredia, A.; Rodríguez, R. Total recovery of the waste of two-phase olive oil processing: Isolation of added-value compounds. J. Agric. Food Chem. 2004, 52, 5849–5855. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Olive mill solid waste biorefinery: High-temperature thermal pre-treatment for phenol recovery and biomethanization. J. Clean. Prod. 2017, 148, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Heredia, A.; Guillén, R.; Jiménez, A. Production in large quantities of highly purified hydroxytyrosol from liquid− solid waste of two-phase olive oil processing or “Alperujo”. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.V.; Boas, L.V. Phenolic compounds and antioxidant activity of Olea europaea L. Fruits and leaves. Food Sci. Technol. Int. 2006, 12, 385–396. [Google Scholar] [CrossRef]
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Briante, R.; Patumi, M.; Terenziani, S.; Bismuto, E.; Febbraio, F.; Nucci, R. Olea europaea L. leaf extract and derivatives: Antioxidant properties. J. Agric. Food Chem. 2002, 50, 4934–4940. [Google Scholar] [CrossRef]
- Bouaziz, M.; Sayadi, S. Isolation and evaluation of antioxidants from leaves of a Tunisian cultivar olive tree. Eur. J. Lipid Sci. Technol. 2005, 107, 497–504. [Google Scholar] [CrossRef]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [Green Version]
- Huertas-Alonso, A.J.; Gonzalez-Serrano, D.J.; Hadidi, M.; Salgado-Ramos, M.; Orellana-Palacios, J.C.; Sánchez-Verdú, M.P.; Xia, Q.; Simirgiotis, M.J.; Barba, F.J.; Nabi Dar, B.; et al. Table Olive Wastewater as a Potential Source of Biophenols for Valorization: A Mini Review. Fermentation 2022, 8, 215. [Google Scholar] [CrossRef]





| Origin | Variety | Processing | Concentration (mg/kg) | Method | References | |||
|---|---|---|---|---|---|---|---|---|
| OLE | TYR | HT | HT-AC | |||||
| Spain | Marfil | Greek-style | 1.40 ± 0.31 | 201.2 ± 23.2 | 384.1 ± 81.2 | 2.85 ± 0.71 | LC-ESI-MS/MS | [17] |
| Azeitera | Spanish-style | 105.9 ± 4.7 | 60.2 ± 5.6 | 605.1 ± 10.6 | n.dr. | RP-HPLC-DAD (OLE) RP-HPLC-FLD (TYR, HT) | [18] | |
| 96.8 ± 6.5 | 54.2 ± 3.9 | 581.2 ± 12.5 | n.dr. | |||||
| Carrasqueña | Spanish-style | 150 ± 10.5 | 75.1 ± 5.2 | 825.3 ± 14.5 | n.dr. | |||
| 138.4 ± 5.9 | 80.2 ± 4.8 | 812.5 ± 11.5 | n.dr. | |||||
| Conserva de Elvas | Spanish-style | 86.4 ± 2.6 | 35.2 ± 5.6 | 415.6 ± 8.5 | n.dr. | |||
| 80.9 ± 4.7 | 39.6 ± 2.1 | 394.1 ± 10.6 | n.dr. | |||||
| Morisca | Spanish-style | 80.5 ± 6.9 | 49.8 ± 4.6 | 398.6 ± 5.8 | n.dr. | |||
| 86.9 ± 2.8 | 45.6 ± 6.4 | 455.9 ± 7.1 | n.dr. | |||||
| Carrasqueña | Spanish-style | 3 ± 1 | 64 ± 10 | 876 ± 82 | n.dr. | RP-HPLC-DAD | [19] | |
| Manzanilla | Spanish-style | 1411.0 ± 452.7 | 78.6 ± 6.4 | 1005.5 ± 25.4 | n.dr. | HPLC-MS | [20] | |
| Hojiblanca | Spanish-style | 96.3 ± 42.8 | 79.1 ± 15.4 | 1133.1 ± 110.6 | n.dr. | |||
| Darkening | n.dr. | 19.5 ± 2.1 | 40.9 ± 6.3 | n.dr. | ||||
| Darkening | n.dr. | 55 ± 3 | 275 ± 10 | n.dr. | HPLC-DAD | [21] | ||
| Italy | Ascolana tenera | Natural-style | 553.4 ± 133 | 97.2 ± 6.5 | 1770.3 ± 324 | n.dr. | RP-HPLC-FLD | [22] |
| Spanish-style | 209.3 ± 8 | 60.7 ± 1.0 | 391.2 ± 8 | n.dr. | ||||
| 156.3 ± 3.9 | 63.1 ± 1.4 | 372 ± 10.1 | n.dr. | |||||
| 89.2 ± 5.2 | 38.5 ± 4.3 | 103.6 ± 8 | n.dr. | |||||
| 77.9 ± 6.7 | 29.7 ± 5.4 | 92.5 ± 11 | n.dr. | |||||
| 225 ± 2.9 | 225.5 ± 13 | 752.5 ± 10 | n.dr. | |||||
| 79.2 ± 2.1 | 71.2 ± 4.2 | 402.1 ± 5.1 | n.dr. | |||||
| 76.8 ± 6.7 | 60.4 ± 8.5 | 324.5 ± 9.4 | n.dr. | |||||
| Leccino | Greek-style | 0.00 | 22.78 ± 6.15 | 150.17 ± 41.20 | n.dr. | UHPLC-DAD | [23] | |
| Bella di Cerignola | Greek-style | n.dr. | 80.5 ± 6.9 | 421.8 ± 30.7 | 26.7 ± 0.9 | HPLC-DAD | [24] | |
| Termite di Bitetto | Greek-style | n.dr. | 28 ± 0.7 | 258.2 ± 12.1 | 0.0 | |||
| Cellina di Nardò | Greek-style | n.dr. | 353.5 ± 26.2 | 1393.3 ± 38 | 0.0 | |||
| Nocellara del Belice | Spanish-style | 35.8 ± 10.3 | 51.5 ± 4.1 | 535.4 ± 37.2 | 62.3 ± 11 | HPLC-MS | [25] | |
| Castelvetrano-style | 49.6 ± 16.5 | 49.0 ± 11.7 | 715.8 ± 79.4 | 26.8 ± 13.8 | ||||
| Portugal | Negrinha de Freixo | California-style | n.dr. | 161.3 ± 15.5 | 672.4 ± 75.4 | n.dr. | RP-HPLC-DAD | [26] |
| Galega | Not mentioned (naturally black) | n.dr. | 139.1 ± 24.0 | 3833.0 ± 180.9 | n.dr. | |||
| California USA | Kalamata | Greek-style | 7.303 | 1.315 | 134.329 | n.dr. | UHPLC-(ESI) MS/MS | [27] |
| Manzanillo | California-style | 0.974 | 0.435 | 19.981 | n.dr. | |||
| Spanish-style | 3.205 | 0.859 | 133.685 | n.dr. | ||||
| Manzanilla | California-style | 36.7 ± 3.1 | n.dr. | 210.0 ± 18.8 | n.dr. | UHPLC-QqQ MS/MS dMRM | [28] | |
| Mission | Dry-salted black olives | 516.2 ± 44.3 | n.dr. | 633.8 ± 55.1 | n.dr. | |||
| Greece | Throuba Thassos | Dry-salted black olives | 1459.5 ± 100.1 | n.dr. | 195.1 ± 7.8 | n.dr. | ||
| Algeria | Azz Sed | Spanish-style | n.dc. | 37.27 ± 0.73 | 105.97 ± 12.2 | n.dr. | RP-HPLC-DAD | [29] |
| Gordal | Spanish-style | n.dc | 43.65 ± 1.09 | 45.68 ± 1.49 | n.dr. | |||
| Sevilla | Spanish-style | n.dc | 106.49 ± 0.26 | 545.42 ± 13.24 | n.dr. | |||
| Sigoise | Spanish-style | 1840.29 ± 49.27 | 35.84 ± 8.76 | 98.80 ± 19.8 | n.dr. | |||
| Teffahi | Spanish-style | n.dc | 24.38 ± 0.00 | 14.49 ± 1.49 | n.dr. | |||
| Bouchouk | Spanish-style | n.dc | 27.60 ± 4.23 | 100.60 ± 1.65 | n.dr. | |||
| Azz Taz | Spanish-style | n.dc | 24.35 ± 0.00 | 38.22 ± 0.00 | n.dr. | |||
| Tunisia | Chétoui | Spanish-style | 307 ± 1.2 | 49 ± 0.2 | 3750 ± 8.3 | n.dr. | HPLC-DAD | [30] |
| 480 ± 2 | 42 ± 0.02 | 2300 ± 9.3 | n.dr. | |||||
| Turkey | Gemlik | Dry-salted black olives | 231 ± 1 | 78 ± 0 | 221 ± 1 | 64 ± 0 | LC-DAD-ESI-MS/MS | [31] |
| Origin | Variety | Category | Concentration (mg/kg) | Method | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HT | TYR | HT-AC | 3,4-DHPEA-EDA | 3,4-DHPEA-EA | p-HPEA-EDA | p-HPEA-EA | OLE | S-DER | |||||
| Calabria Italy | Frantoio | EVOO | 5.3 ± 0.9 | 5.4 ± 0.7 | n.dr. | 98.4 ± 3.5 | 27.5 ± 1.6 | 52.9 ± 1.7 | 4.4 ± 0.9 | n.dr. | n.dr. | HPLC -DAD | [32] |
| Calabria Italy | 4.6 ± 0.7 | 2.5 ± 0.3 | 1.9 ± 0.3 | 50.3 ± 2.8 | 22.5 ± 2.2 | 34.9 ± 1.7 | 3.0 ± 0.9 | n.dr. | n.dr. | ||||
| Calabria Italy | 1.2 ± 0.4 | 1.1 ± 0.2 | n.d. | 89.4 ± 2.9 | 19.0 ± 1.7 | 37.9 ± 1.6 | n.d. | n.dr. | n.dr. | ||||
| Praia a mare, Calabria Italy | 1.7 ± 0.1 | 1.7 ± 0.5 | 0.7 ± 0.1 | 56.0 ± 3.1 | 18.9 ± 1.9 | 34.6 ± 2.7 | 1.7 ± 0.9 | n.dr. | n.dr. | ||||
| Italy | Coratina | VOO | 1.97–7.1 | 5.7–16.4 | n.dr. | 65.1–147.7 | 94.6–268.9 | 89.4–153.7 | 25.4–120.8 | n.dr. | n.dr. | IOC Extraction RP-HPLC-DAD | [33] |
| Bosana | 1.9–3.0 | 3.7–5.7 | n.dr. | 71.8–237.2 | 53.4–118.7 | 70.8–155.6 | 16.1–34.7 | n.dr. | n.dr. | ||||
| Semidana | 1.3–4.8 | 2.7–9.2 | n.dr. | 4.5–90.8 | 11.6–66.2 | 17.0–57.5 | 5.5–17.2 | n.dr. | n.dr. | ||||
| Tonda di Cagliari | 0.9–5.3 | 2.2–10.4 | n.dr. | 13.5–138.8 | 13.7–59.9 | 23.2–108.0 | 8.4–26.3 | n.dr. | n.dr. | ||||
| Tuscany Italy | Multi- varietal | EVOO | 161.5 ± 4.5 | 122.5 ± 3.5 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | Acid hydrolysis HPLC-DAD | [34] |
| Northern Italy | Frantoio | EVOO | 0.9 ± 0.0 | 4.2 ± 0.6 | 1.3 ± 0.2 | 76.7 ± 55 | 7.9 ± 0.4 | 6.7 ± 0.9 | n.dr. | 18.5 ± 1.6 | n.dr. | LLE HPLC-DAD-MS | [35] |
| 3.0 ± 0.2 | 4.3 ± 0.3 | 1.9 ± 0.2 | 62.2 ± 4.9 | 2.6 ± 0.3 | 2.2 ± 0.1 | n.dr. | 5.3 ± 0.3 | n.dr. | |||||
| Casaliva | 0.7 ± 0.7 | 3.1 ± 0.4 | 1.8 ± 0.2 | 56.6 ± 4.7 | 3.2 ± 0.1 | 7.4 ± 0.4 | n.dr. | 6.6 ± 0.7 | n.dr. | ||||
| 1.9 ± 0.3 | 4.6 ± 0.4 | 1.5 ± 0.2 | 55.0 ±0.8 | 1.4 ± 0.0 | 2.7 ± 0.2 | n.dr. | 1.8 ± 0.1 | n.dr. | |||||
| Organic Casaliva | 2.3 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.3 | 108.2 ± 9.6 | 9.2 ± 0.9 | 12.3 ± 1.2 | n.dr. | 12.8 ± 1.9 | n.dr. | ||||
| 4.3 ± 0.3 | 4.9 ± 0.8 | 3. ± 0.23 | 98.9 ± 13.3 | 3.5 ± 0.1 | 7.3 ± 0.1 | n.dr. | 2.7 ± 0.2 | n.dr. | |||||
| Multi-varietal | 6.4 ± 0.2 | 2.5 ± 0.4 | 3.4 ± 0.2 | 102.4 ± 4.6 | 5.5 ± 0.1 | 10.9 ± 1.0 | n.dr. | 10.0 ± 1.1 | n.dr. | ||||
| 0.3 ± 0.0 | 1.1 ± 0.1 | 2.9 ± 0.2 | 54.6 ± 3.0 | 6.4 ± 0.6 | 0.9 ± 0.0 | n.dr. | 1.5 ± 0.1 | n.dr. | |||||
| Organic multi-varietal | 11.4 ± 0.6 | 5.6 ± 0.6 | 1.7 ± 0.1 | 106.9 ± 12.6 | 8.8 ± 2.9 | 12.5 ± 0.6 | n.dr. | 15.4 ± 1.5 | n.dr. | ||||
| 0.6 ± 0.0 | 2.8 ± 0.4 | 1.9 ± 0.3 | 89.4 ± 1.3 | 2.2 ± 0.1 | 14.4 ± 1.3 | n.dr. | 9.5 ± 0.7 | n.dr. | |||||
| Salerno Italy | N/D (Cilento PDO) | VOO | 37–41.3 | 23.8–34.6 | n.dr. | n.dr. | 19.9–24.9 | n.dr. | 23.8–35 | 120.4–140 | n.dr. | RP-HPLC-DAD | [36] |
| Abruzzo Italy | Gentile | VOO | 1.6–3.7 | 3.9–5.8 | n.dr. | n.dr. | 20.8–22.0 | n.dr. | n.dr. | n.dr. | 232.8–359.9 | HPLC- DAD HPLC- MS | [37] |
| 0.8 ± 0.1 | 4.1 ± 0.2 | n.dr. | n.dr. | 15.2 ± 0.3 | n.dr. | n.dr. | n.dr. | 173.8 ± 3.0 | |||||
| Leccino | 2.3 ± 0.1 | 6.1 ± 0.2 | n.dr. | n.dr. | 5.9 ± 0.1 | n.dr. | n.dr. | n.dr. | 91.0 ± 1.6 | ||||
| n.d. | 4.0 ± 0.2 | n.dr. | n.dr. | 5.4 ± 0.2 | n.dr. | n.dr. | n.dr. | 120.1 ± 1.4 | |||||
| Dritta | 2.0–2.8 | 7.4–7.9 | n.dr. | n.dr. | 14.9–30.9 | n.dr. | n.dr. | n.dr. | 189.9–299.1 | ||||
| 3.5 ± 0.1 | 8.8 ± 0.24 | n.dr. | n.dr. | 11.3 ± 0.2 | n.dr. | n.dr. | n.dr. | 310.3 ± 5.3 | |||||
| Southwest Spain | Arbequina | VOO | 1.3 ± 0.4 | 1.9 ± 0.3 | 42.3 ± 3.6 | 75.5 ± 23.9 | 33.7 ± 1.9 | 59.9 ± 6.9 | 16.5 ± 0.9 | n.dr. | n.dr. | SPE RP-HPLC-DAD | [38] |
| 0.4 ± 0.0 | 0.6 ± 0.0 | 36.8 ± 1.5 | 44.7 ± 1.1 | 104.4 ± 1.2 | 36.8 ± 0.2 | 9.7 ± 1.8 | n.dr. | n.dr. | |||||
| Carrasqueña | 2.8 ± 0.8 | 3.5 ± 0.9 | 7.3 ± 0.3 | 141.1 ± 1.1 | 68.4 ± 14.9 | 73.5 ± 18.9 | 82.9 ± 6.9 | n.dr. | n.dr. | ||||
| 0.6 ± 0.0 | 3.4 ± 0.0 | 6.9 ± 1.4 | 88.7 ± 10.5 | 95.2 ± 33.4 | 36.1 ± 3.1 | 14.3 ± 1.7 | n.dr. | n.dr. | |||||
| Corniche | 2.0 ± 0.8 | 3.1 ± 1.9 | n.d. | 69.0 ± 14.7 | 61.2 ± 16.5 | 101.7 ± 21.5 | 12.4 ± 2.3 | n.dr. | n.dr. | ||||
| 0.9 ± 0.3 | 2.1 ± 1.0 | 20.9 ± 2.5 | 150.5 ± 12.6 | 37.0 ± 9.3 | 116.9 ± 32.4 | 10.5 ± 3.4 | n.dr. | n.dr. | |||||
| Manzanilla Cacereña | 1.5 ± 0.6 | 5.9 ± 1.4 | n.dc. | 56.0 ± 9.6 | 32.8 ± 9.8 | 58.2 ± 7.3 | 73.4 ± 0.9 | n.dr. | n.dr. | ||||
| 0.7 ± 0.3 | 7.8 ± 1.5 | n.dr. | 40.6 ± 4.5 | 48.3 ± 5.9 | 59.4 ± 8.1 | 10.9 ± 3.1 | n.dr. | n.dr. | |||||
| Morisca | 1.3 ± 0.3 | 3.0 ± 0.9 | 6.2 ± 10.0 | 70.6 ± 7.2 | 30.4 ± 4.1 | 41.4 ± 31.6 | 51.0 ± 1.2 | n.dr. | n.dr. | ||||
| 1.4 ± 0.7 | 3.2 ± 1.4 | 43.3 ± 8.3 | 42.5 ± 5.3 | 18.9 ± 0.8 | 40.3 ± 3.6 | 23.9 ± 5.0 | n.dr. | n.dr. | |||||
| Picual | 3.3 ± 1.2 | 5.7 ± 1.3 | n.dc. | 89.3 ± 15.6 | 73.3 ± 16.9 | 44.2 ± 3.5 | 39.8 ± 7.4 | n.dr. | n.dr. | ||||
| 1.6 ± 0.8 | 3.5 ± 2.7 | n.dc. | 71.7 ± 3.9 | 46.9 ± 0.8 | 39.7 ± 7.5 | 68.8 ± 9.6 | n.dr. | n.dr. | |||||
| Verdial de Badajoz | 1.4 ± 0.2 | 6.3 ± 0.6 | n.dr. | 143.8 ± 34.2 | 33.7 ± 5.7 | 166.4 ± 15.0 | 41.6 ± 11.8 | n.dr. | n.dr. | ||||
| 0.8 ± 0.4 | 4.2 ± 1.1 | n.dr. | 71.6 ± 18.8 | 16.5 ± 1.7 | 123.5 ± 19.1 | 6.5 ± 0.5 | n.dr. | n.dr. | |||||
| Jaén, Spain | Picual | VOO | 2.8–7.8 | 4.9–9.9 | n.dr. | 221.4–849.7 | 29.5–929.2 | 19.5–248.9 | 24.4–344.2 | n.dr. | n.dr. | LLE HPLC- DAD | [39] |
| 2.8–6.2 | 0.9–7.8 | n.dr. | 231.5–788.0 | 26.2–790.7 | 13.5–262.2 | 22.0–269.5 | n.dr. | n.dr. | |||||
| Seville, Spain | Arbequina Hojiblanca Manzanilla Picual N/A | EVOO | 50–200 | 40–180 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | Acid hydrolysis HPLC- UV-FL | [40] |
| N/D | OO | 5-20 | 5–30 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | |||
| Spain | Picual-Arbequina blend | VOO | 15.7 ± 0.9 | 9.1 ± 0.5 | n.dr. | n.dr. | n.dr. | n.dr. | 38.5 ± 3.8 | n.dr. | n.dr. | SPE HPLC-ESI-TOF/MS | [41] |
| 14.3 ± 0.2 | 8.9 ± 0.3 | n.dr. | n.dr. | n.dr. | n.dr. | 44.8 ± 0.9 | n.dr. | n.dr. | SPE HPLC-UV | ||||
| Catalonia Spain | Arbequina | VOO | 2.5 | 3.0 | 1.6 | 152 | 68 | 20 | 42 | n.dr. | 26.4 | LLE UPLC-MS/MS | [42] |
| Spain | Cornicabra | EVOO | 0.9–2.8 | 1.0–2.3 | n.dr. | 396–770 | 136–301 | 228–498 | 39–138 | n.dr. | n.dr. | SPE HPLC-DAD | [43] |
| Messenia, Greece | Koroneiki | EVOO | 4.1 ± 0.1 | 0.4 ± 0.0 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | Selective ion monitoring GC/MS | [44] |
| 9.3 ± 0.1 | 0.4 ± 0.0 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | |||||
| 20.2 ± 1.0 | 0.4 ± 0.1 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | |||||
| Morocco | Arbequina | VOO | 6.4 ± 2.7 | 11.6 ± 3.3 | 26.6 ± 32.7 | n.dr. | 55.4 ± 6.4 | n.dr. | 53.9 ± 4.4 | n.dr. | n.dr. | LLE LC-ESI- IT-MS | [45] |
| Arbosana | 1.3 ± 1.9 | 3.3 ± 4.0 | 1.5 ± 1.3 | n.dr. | 21.0 ± 8.4 | n.dr. | 18.9 ± 2.2 | n.dr. | n.dr. | ||||
| Cornicabra | 4.3 ± 0.4 | 6.6 ± 0.4 | 1.4 ± 0.1 | n.dr. | 96.9 ± 14.5 | n.dr. | 93.9 ± 6.4 | n.dr. | n.dr. | ||||
| Frantoio | 0.8 ± 0.4 | 4.1 ± 0.6 | 0.3 ± 0.2 | n.dr. | 24.5 ± 14.6 | n.dr. | 34.8 ± 8.7 | n.dr. | n.dr. | ||||
| Hojiblanca | 0.5 ± 0.3 | 5.4 ± 2.1 | 1.1 ± 0.8 | n.dr. | 35.8 ± 17.1 | n.dr. | 41.1 ± 18.1 | n.dr. | n.dr. | ||||
| Koroneiki | 8.3 ± 2.6 | 7.5 ± 2.4 | 2.2 ± 0.3 | n.dr. | 93.6 ± 9.0 | n.dr. | 66.1 ± 15.8 | n.dr. | n.dr. | ||||
| Manzanilla | 3.8 ± 3.3 | 10.8 ± 5.6 | 1.8 ± 1.5 | n.dr. | 54.0 ± 33.2 | n.dr. | 65.6 ± 37.1 | n.dr. | n.dr. | ||||
| P-Languedoc | 2.2 ± 0.8 | 9.5 ± 1.2 | 0.8 ± 0.2 | n.dr. | 46.6 ± 10.9 | n.dr. | 46.3 ± 14.4 | n.dr. | n.dr. | ||||
| P-Marocaine | 2.2 ± 1.7 | 8.5 ± 1.6 | 0.9 ± 0.3 | n.dr. | 35.2 ± 7.6 | n.dr. | 35.9 ± 3.7 | n.dr. | n.dr. | ||||
| Picual | 4.8 ± 2.7 | 9.2 ± 2.0 | 2.2 ± 1.9 | n.dr. | 51.96 ± 26.81 | n.dr. | 47.4 ± 6.1 | n.dr. | n.dr. | ||||
| Dahbia | 0.2 ± 0.0 | 1.7 ± 0.2 | 0.2 ± 0.0 | n.dr. | 26.1 ± 0.4 | n.dr. | 33.1 ± 0.9 | n.dr. | n.dr. | ||||
| Haouzia | 3.7 ± 1.4 | 7.6 ± 2.0 | 0.5 ± 0.3 | n.dr. | 63.5 ± 12.7 | n.dr. | 51.1 ± 22.8 | n.dr. | n.dr. | ||||
| Menara | 4.1 ± 2.7 | 11.4 ± 2.6 | 0.4 ± 0.2 | n.dr. | 41.6 ± 10.5 | n.dr. | 43.8 ± 5.3 | n.dr. | n.dr. | ||||
| Algeria | Azeradj | EVOO | 2.8–3.0 | 13.3–26.4 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 1.2–2.1 | n.dr. | SPE HPLC- UV-vis | [46] |
| Mekki | 1.1–1.4 | 9.0–11.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Neb djemel | 4.4–7.3 | 15.1–17.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Chemlal | 3.3–4.2 | 13.6–19.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.0–4.4 | n.dr. | ||||
| Hamra | 1.1–4.1 | 9.9–20.7 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 8.3–13.1 | n.dr. | ||||
| Blanquette de Guelma | 4.8–8.9 | 19.5–21.6 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 1.3–2.2 | n.dr. | ||||
| Limli | 2.9–3.2 | 13.1–18.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Aberkane1 | 1.1–1.2 | 16.7–18.5 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Aimell | 2.2–6.3 | 18.0–18.3 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Rougette de la mitidja | 4.1–4.6 | 14.6–19.6 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 1.2–1.5 | n.dr. | ||||
| Aghenaou | 1.7–2.4 | 14.0–18.1 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Boughenfas | 2.6–2.8 | 16.6–26.4 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 1.6–2.5 | n.dr. | ||||
| Bouichret | 3.1–4.2 | 13.5–14.6 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Aghenfas | 3.2–3.9 | 18.4–25.1 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.8–5.2 | n.dr. | ||||
| Bouchouk de Guergour | 3.8–4.2 | 14.5–20.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.3–0.5 | n.dr. | ||||
| X-Aghenfas | 3.5–8.7 | 13.9–36.3 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Bounguergueb | 1.8–3.5 | 13.9–14.0 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Ronde de miliana | 0.0–1.5 | 16.6–20.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 1.0–1.5 | n.dr. | ||||
| Sigoise | 1.4–2.6 | 22.4–27.9 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.2–0.4 | n.dr. | ||||
| Grosse du Hamma | 9.67-14.93 | 28.1–32.9 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.4–0.9 | n.dr. | ||||
| Rougette de Guelma | 3.3–3.7 | 14.0–18.5 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.7–1.4 | n.dr. | ||||
| Tunisia | Oueslati | EVOO | 3.8–7.2 | 1.9–3.1 | 0.3–1.3 | n.dr. | 222.6–537.8 | n.dr. | 2.9–19.4 | n.dr. | n.dr. | RRLC- ESI- TOF-MS | [47] |
| Hatay Turkey | Halhali | VOO | 5.5 ± 0.0 | 10.3 ± 0.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | LLE HPLC- DAD | [48] |
| EVOO | 5.2 ± 0.9 | 14.8 ± 0.4 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Balikesir Turkey | Ayvalik | VOO | 0.1–0.8 | 0.7–1.1 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | HPLC- DAD | [49] |
| Domat | 0.0–1.2 | 0.2–0.9 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Gemlik | 0.2–0.4 | 0.5–1.6 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Origin | Variety | Year | Concentration (mg/L) | Method | References | |
|---|---|---|---|---|---|---|
| TYR | HT | |||||
| France | Not specified | n.dr. | 0.0092 | LC-MS/MS | [50] | |
| Australia | - | 0.0054 | ||||
| China | 0.0056 | |||||
| China | 0.000071 | |||||
| Salento, Italy | Negroamaro (Red wine) | - | n.dr. | 2.3 ± 0.8 mg/kg | HPLC | [51] |
| Primitivo (Red wine) | - | 2.7 ± 0.7 mg/kg | ||||
| Croatia | Cabernet Sauvignon (Red wine) | 2013 | 44.5 | 2.3 | UV/VIS-HPLC | [52] |
| 2014 | 29.8 | 2.1 | ||||
| 2015 | 46.4 | 1.746 | ||||
| Merlot (Red wine) | 2013 | 40.7 | 2.7 | |||
| 2014 | 41.5 | 3.2 | ||||
| 2015 | 36.9 | 2.6 | ||||
| Plavac mali (Red wine) | 2013 | 41.3 | 2.9 | |||
| 2014 | 31.1 | 2.4 | ||||
| 2015 | 48.3 | 2.6 | ||||
| Teran (Red wines) | 2013 | 40.6 | 3.7 | |||
| 2014 | 21.6 | 4.0 | ||||
| 2015 | 36.5 | 3.4 | ||||
| Jerez de la Frontera, Spain | Corredera (White wine) | - | n.dr. | 0.173 | HPLC | [53] |
| Moscatel (White wine) | 0.159 | |||||
| Chardonnay (White wine) | 0.167 | |||||
| Sauvignon Blanc (White wine) | 0.288 | |||||
| Palomino Fino (White wine) | 0.089 | |||||
| Vijiriega (White wine) | 0.238 | |||||
| Spain | Bach Viña Extrísimo (White wine) | 2016 | 10.4 | 1.3 | LC/MS-MS | [54] |
| Girona, Spain | Jardins Negre (Red wine) | 2017 | 25.30 | 1.80 | LC/MS-MS | [55] |
| Italy | Greco di Tufo (White wine) | 1998 | 1.1 | 2.7 | HPLC | [56] |
| Verdicchio (White wine) | 1998 | 3.0 | 1.6 | |||
| Pinot Grigio (White wine) | 1997 | 2.3 | 1.9 | |||
| Blended | 1998 | 5 | 6.1 | |||
| 1996 | 6 | 5.9 | ||||
| Barbera (Red wine) | - | 5.9 | 9.6 | |||
| Montepulciano (Red wine) | 1998 | 5.9 | 0.5 | |||
| Italy | White wine | 1.42–2.34 | 1.79–2.00 | GC-MS | [57] | |
| Red wine | 3.61–4.80 | 3.81–4.37 | ||||
| Jerez de la Frontera, Spain | Tempranillo (Red wine) | 2009 | 20.51–22.76 | 0.82–1.84 | HPLC-FD | [58] |
| Blasco (Red wine) | 27.38 | 1.55 | ||||
| Cabernet Sauvignon (Red wine) | 31.95–32.57 | 5.02–3.63 | ||||
| Petit Verdot (Red wine) | 40.59–38.50 | 4.12–2.33 | ||||
| Syrah (Red Wine) | 2010 | 40.98–34.11 | 1.11–0.82 | |||
| Merlot (Red Wine) | 44.46 | 1.77 | ||||
| Tintilla de Rota (Red Wine) | 28.91–30.97 | 2.66–1.65 | ||||
| Melonera (Red Wine) | 35.31–36.86 | 0.53–0.45 | ||||
| Tempranillo (Red Wine) | 25.03–44.26 | 1.78 | ||||
| Vitis silvestris (Red Wine) | 20.38–40.20 | 0.28–3.09 | ||||
| Palomino negro (Red Wine) | 29.57 | 1.10 | ||||
| Rome (Red Wine) | 35.41–35.57 | 1.67–1.86 | ||||
| Garnacha (Red Wine) | 26.73–30.15 | 1.02–1.28 | ||||
| Country | Name of the Dietary Survey | Period of Survey | Nº of Subjects |
|---|---|---|---|
| Austria | Austrian Study on Nutritional Status 2010–2012—Adults (ASNS–Adults) | 2010–2012 | 615 |
| Belgium | Belgian National Food consumption survey (NATIONAL-FCS-2014) | 2014–2015 | 2278 |
| Croatia | Croatian food consumption survey on adults (NIPNOP-HAH-2011-2012) | 2011–2012 | 2000 |
| Czech Republic | Czech National Food Consumption Survey (SISP04) | 2003–2004 | 1666 |
| Cyprus | National dietary survey of the adult population of Cyprus (CY 2014-2017-LOT2) | 2014–2017 | 812 |
| Denmark | The Danish National Dietary survey 2005–2008 (DANSDA 2005-08) | 2005–2008 | 1739 |
| Estonia | National Dietary Survey among 11–74 years old individuals in Estonia (DIET-2014-EST-A) | 2013–2015 | 2124 |
| Finland | National FINDIET 2012 Survey (FINDIET2012) | 2012 | 1295 |
| France | The French national dietary survey (INCA3) | 2014–2015 | 1773 |
| Germany | National Nutrition Survey II | 2007 | 10,419 |
| Greece | The EFSA-funded collection of dietary or related data in the general population aged 10–74 years in Greece (GR-EFSA-LOT2 2014-2015) | 2014–2016 | 791 |
| Hungary | National Repr Surv (NATIONAL REPR SURV) | 2003 | 1074 |
| Ireland | National Adult Nutrition Survey (NANS 2012) | 2008–2010 | 1274 |
| Italy | Italian National Food Consumption Survey (INRAN SCAI 2005-06) | 2005–2006 | 2313 |
| Latvia | Latvian National Dietary survey (LATVIA_2014) | 2012–2015 | 1080 |
| Netherlands | Dutch National food consumption survey 2012–2016 (FCS2016_CORE) | 2012–2017 | 4313 |
| Portugal | National Food, Nutrition and Physical Activity Survey of the Portuguese general population (IAN.AF 2015-2016) | 2015–2016 | 3102 |
| Romania | Dietary Pilot Adults | 2012 | 1254 |
| Slovenia | Slovenian national food consumption survey (SI. MENU-2018) | 2017–2018 | 2119 |
| Spain | Spanish National dietary survey in adults, elderly, and pregnant woman (ENALIA2) | 2013–2015 | 669 |
| Sweden | Swedish National Dietary Survey—Riksmaten adults 2010–11 (RIKSMATEN 2010) | 2010–2011 | 1430 |
| United Kingdom | National Diet and Nutrition Survey (NDNS) | 2000–2001 | 1724 |
| Health Benefit | Mechanism of Action | References |
|---|---|---|
| Anti-inflammatory | Inhibition of LPS mediated expression of TNF-α and IL-1β ↓ Expression of NADPH oxidase and MAPKs ↓ Inflammasome | [5,6] |
| Neuroprotective | Inhibition of the formation of α-synuclein and β-amyloid fibrils | [7,8,9] |
| Antiangiogenic | Inhibition of VEGF receptor-2 activation | [10] |
| Pro-apoptotic | ↓ Proliferation of MCF-7 | [13,14] |
| Anti-diabetic | Insulin-like effect on target cells | [61,62] |
| Antioxidant | Blood lipids’ protection from oxidative stress | [15] |
| Foods under Study | Food Consumption | Free Hydroxytyrosol Daily Intake from Foods under Study | HT from Oleuropein and Aglycone |
|---|---|---|---|
| Range of Means among EU Surveys (g/day) | Range of Means (mg/day) | Range of Means (mg/day) | |
| EVOO/VOO | 0–33.78 | 0–0.18 | 0–1.08 |
| Table olives | 0.02–3.41 | 0.010–2.14 | 0.03–6.42 |
| Wine | 10.65–89.08 | 0.022–0.186 | - |
| Food | Quantity | Origin | References |
|---|---|---|---|
| Yogurt | 5 mg HT/120 g (1 unit = 120 g) | Olive Pomace Liquid-enriched powder (LOPP) and pulp-enriched powder (POPP) obtained from olive pomace | [93] |
| Fuet (Dried cured sausage) | 200 mg/kg | Synthetic | [94] |
| Fuet (Dried cured sausage) | 200 mg/kg | Olive vegetation waters of olive | [94] |
| Cookies | 5.25 mg/30 g | Olive oil wastewaters | [95] |
| Smoothies | 5.78 mg HT + OLE/100 g | Olive leaf extracts | [96] |
| Chicken sausage | 50 mg/kg | Olive water/olive leaves | [97] |
| Blood orange juice | 28.8–57.6 mg/L | Olive mill wastewater | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo-Fernández, M.; Gonzalez-Ramirez, M.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Hydroxytyrosol in Foods: Analysis, Food Sources, EU Dietary Intake, and Potential Uses. Foods 2022, 11, 2355. https://doi.org/10.3390/foods11152355
Gallardo-Fernández M, Gonzalez-Ramirez M, Cerezo AB, Troncoso AM, Garcia-Parrilla MC. Hydroxytyrosol in Foods: Analysis, Food Sources, EU Dietary Intake, and Potential Uses. Foods. 2022; 11(15):2355. https://doi.org/10.3390/foods11152355
Chicago/Turabian StyleGallardo-Fernández, Marta, Marina Gonzalez-Ramirez, Ana B. Cerezo, Ana M. Troncoso, and M. Carmen Garcia-Parrilla. 2022. "Hydroxytyrosol in Foods: Analysis, Food Sources, EU Dietary Intake, and Potential Uses" Foods 11, no. 15: 2355. https://doi.org/10.3390/foods11152355
APA StyleGallardo-Fernández, M., Gonzalez-Ramirez, M., Cerezo, A. B., Troncoso, A. M., & Garcia-Parrilla, M. C. (2022). Hydroxytyrosol in Foods: Analysis, Food Sources, EU Dietary Intake, and Potential Uses. Foods, 11(15), 2355. https://doi.org/10.3390/foods11152355