In Vitro Release of Anthocyanins from Microencapsulated Natal Plum (Carissa macrocarpa) Phenolic Extract in Alginate/Psyllium Mucilage Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Natal Plum Phenolic Extraction and Alginate Beads Preparation
2.3. Total Anthocyanin Content and Encapsulation Efficiency
2.4. Microstructure of the Beads
2.5. Thermogravimetric Analysis (TGA)
2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
2.7. In Vitro Release Behavior of Anthocyanins
2.8. Statistical Analysis
3. Results
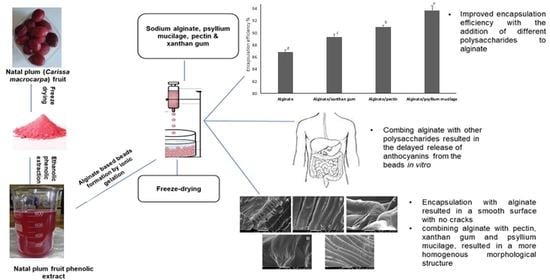
3.1. The Effect of Different Microencapsulation Wall Materials on Encapsulation Efficiency
3.2. Impact of Encapsulation on the Surface Morphology of the Beads
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.4. The Effect of Encapsulation on the Thermal Stability of Anthocyanins in Encapsulated Alginate-Based Beads
3.5. In Vitro Release Behavior of Anthocyanins
3.6. Effect of Gastrointestinal Fluids on the Total Anthocyanin Content and Color of the Alginate-Based Beads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jurić, S.; Jurić, M.; Król-Kilińska, Ż.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2020, 36, 1–56. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks, and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rubio, A.; Gavara, R.; Lagaron, J.M. Bioactive packaging: Turning foods into healthier foods through biomaterials. Trends Food Sci. Technol. 2006, 17, 567–575. [Google Scholar] [CrossRef]
- Leong, J.Y.; Lam, W.H.; Ho, K.W.; Voo, W.P.; Lee, M.F.X.; Lim, H.P.; Tey, B.-T.; Poncelet, D.; Chan, E.S. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.G.; Cazarin, C.B.B.; Junior, M.R.M. Anthocyanins: New techniques and challenges in microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef]
- Fernandes, A.; Brás, N.F.; Mateus, N.; de Freitas, V. Understanding the molecular mechanism of anthocyanin binding to pectin. Langmuir 2014, 30, 8516–8527. [Google Scholar] [CrossRef]
- Seke, F.; Manhivi, V.E.; Shoko, T.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Extraction optimisation, hydrolysis, antioxidant properties and bioaccessibility of phenolic compounds in Natal plum fruit (Carissa macrocarpa). Food Biosci. 2021, 44, 101425. [Google Scholar] [CrossRef]
- Ndou, A.; Tinyani, P.P.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. An integrated approach for harvesting Natal plum (Carissa macrocarpa) for quality and functional compounds related to maturity stages. Food Chem. 2019, 293, 499–510. [Google Scholar] [CrossRef]
- Hosseini, H.; Jafari, S.M. Introducing nano/microencapsulated bioactive ingredients for extending the shelf-life of food products. Adv. Colloid Interface Sci. 2020, 282, 102210. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Duan, M.; Hou, D.; Chen, X.; Shi, J.; Zhou, W. Fabrication and characterization of Ca (II)-alginate-based beads combined with different polysaccharides as vehicles for delivery, release and storage of tea polyphenols. Food Hydrocoll. 2021, 112, 106274. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Navarro, P.; Vallejo, A.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes. Food Chem. 2016, 190, 614–621. [Google Scholar] [CrossRef]
- Mendes, D.D.C.S.; Asquieri, E.R.; Batista, R.D.; de Morais, C.C.; Ascheri, D.P.R.; de Macêdo, I.Y.L.; de Souza Gil, E. Microencapsulation of jabuticaba extracts (Myrciaria cauliflora): Evaluation of their bioactive and thermal properties in cassava starch biscuits. LWT 2021, 137, 110460. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Jarzębski, M.; Pratap-Singh, A.; Guo, Y.; Abd-Manap, Y. Encapsulation of betacyanins from the peel of red dragon fruit (Hylocereus polyrhizus L.) in alginate beads. Food Hydrocoll. 2021, 113, 106535. [Google Scholar] [CrossRef]
- Marefati, A.; Bertrand, M.; Sjöö, M.; Dejmek, P.; Rayner, M. Storage and digestion stability of encapsulated curcumin in emulsions based on starch granule Pickering stabilization. Food Hydrocoll. 2017, 63, 309–320. [Google Scholar] [CrossRef]
- Flamminii, F.; Paciulli, M.; Di Michele, A.; Littardi, P.; Carini, E.; Chiavaro, E.; Pittia, P.; Di Mattia, C.D. Alginate-based microparticles structured with different biopolymers and enriched with a phenolic-rich olive leaves extract: A physicochemical characterization. Curr. Res. Food Sci. 2021, 4, 698–706. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Bušić, A.; Barišić, L.; Vrsaljko, D.; Karlović, S.; Špoljarić, I.; Komes, D. Emulsion templated microencapsulation of dandelion (Taraxacum officinale L.) polyphenols and β-carotene by ionotropic gelation of alginate and pectin. Food Hydrocoll. 2016, 57, 139–152. [Google Scholar] [CrossRef]
- Chen, L.; Ge, M.D.; Zhu, Y.J.; Song, Y.; Cheung, P.C.; Zhang, B.B.; Liu, L.M. Structure, bioactivity and applications of natural hyperbranched polysaccharides. Carbohydr. Polym. 2019, 223, 115076. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V.R.; Zibadi, S. (Eds.) Wheat and Rice in Disease Prevention and Health: Benefits, Risks and Mechanisms of Whole Grains in Health Promotion; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Opanasopit, P.; Apirakaramwong, A.; Ngawhirunpat, T.; Rojanarata, T.; Ruktanonchai, U. Development and characterization of pectinate micro/nanoparticles for gene delivery. AAPS Pharmscitech 2008, 9, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lv, Y. Degradation kinetic of anthocyanins from rose (Rosa rugosa) as prepared by microencapsulation in freeze-drying and spray-drying. Int. J. Food Prop. 2019, 22, 2009–2021. [Google Scholar] [CrossRef]
- Ntenga, R.; Pagore, F.D.; Pizzi, A.; Mfoumou, E.; Ohandja, L.M.A. Characterization of Tannin-Based Resins from the Barks of Ficus platyphylla and of Vitellaria paradoxa: Composites’ Performances and Applications. Mater. Sci. Appl. 2017, 8, 899. Available online: http://www.scirp.org/journal/PaperInformation.aspx?PaperID=80423&#abstract (accessed on 10 July 2022).
- Favaro, L.I.; Balcão, V.M.; Rocha, L.K.; Silva, E.C.; Oliveira, J.M., Jr.; Vila, M.M.; Tubino, M. Physicochemical characterization of a crude anthocyanin extract from the fruits of Jussara (Euterpe edulis Martius): Potential for food and pharmaceutical applications. J. Braz. Chem. Soc. 2018, 29, 2072–2088. [Google Scholar] [CrossRef]
- Kalušević, A.M.; Lević, S.M.; Čalija, B.R.; Milić, J.R.; Pavlović, V.B.; Bugarski, B.M.; Nedović, V.A. Effects of different carrier materials on physicochemical properties of microencapsulated grape skin extract. J. Food Sci. Technol. 2017, 54, 3411–3420. [Google Scholar] [CrossRef]
- Özacar, M.; Soykan, C.; Şengïl, İ.A. Studies on synthesis, characterization, and metal adsorption of mimosa and valonia tannin resins. J. Appl. Polym. Sci. 2006, 102, 786–797. [Google Scholar] [CrossRef]
- Bellary, A.N.; Indiramma, A.R.; Prakash, M.; Baskaran, R.; Rastogi, N.K. Anthocyanin infused watermelon rind and its stability during storage. Innov. Food Sci. Emerg. Technol. 2016, 33, 554–562. [Google Scholar] [CrossRef]
- Tomoda, B.T.; Yassue-Cordeiro, P.H.; Ernesto, J.V.; Lopes, P.S.; Péres, L.O.; da Silva, C.F.; de Moraes, M.A. Characterization of biopolymer membranes and films: Physicochemical, mechanical, barrier, and biological properties. Biopolm. Membr. Films 2020, 67–95. [Google Scholar] [CrossRef]
- De Freitas, E.D.; Rosa, P.C.P.; da Silva, M.G.C.; Vieira, M.G.A. Development of sericin/alginate beads of ketoprofen using experimental design: Formulation and in vitro dissolution evaluation. Powder Technol. 2018, 335, 315–326. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Tohamy, H.-A.S.; Salama, A.; Kamel, S. Thermal properties of carboxymethyl cellulose acetate butyrate. Cellul. Chem. Technol. 2019, 53, 667–675. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Yu, H.-Y.; Liu, Y.-Z.; Qin, Z.; Liu, H.-M.; Ma, Y.-X.; Wang, X.-D. Isolation and structural characterization of cell wall polysaccharides from sesame kernel. LWT 2022, 163, 113574. [Google Scholar] [CrossRef]
- Kang, J.; Guo, Q.; Cui, S.W. Other emerging gums: Flaxseed gum, yellow mustard gum, and psyllium gums. In Handbook of Hydrocolloids; Woodhead Publishing: Sawston, UK, 2021; pp. 597–624. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Koch, L.; Rentschler, C.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Effect of molecular weight reduction, acetylation and esterification on the emulsification properties of citrus pectin. Food Biophys. 2015, 10, 217–227. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Chen, L. Encapsulation of anthocyanin in liposomes using supercritical carbon dioxide: Effects of anthocyanin and sterol concentrations. J. Funct. Foods 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch-based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Sungthongjeeh, S. Modification of theophylline release with alginate gel formed in hard capsules. AAPS Pharmscitech 2007, 8, E1–E8. [Google Scholar] [CrossRef]
- Park, S.A.; Ahn, J.B.; Choi, S.H.; Lee, J.S.; Lee, H.G. The effects of particle size on the physicochemical properties of optimized astaxanthin-rich Xanthophyllomyces dendrorhous-loaded microparticles. LWT-Food Sci. Technol. 2014, 55, 638–644. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.K.; Moon, J.Y.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Comparative antioxidant and antiproliferative activities of red and white pitayas and their correlation with flavonoid and polyphenol content. J. Food Sci. 2011, 76, C38–C45. [Google Scholar] [CrossRef]
- da Silva Carvalho, A.G.; da Costa Machado, M.T.; Barros, H.D.D.F.Q.; Cazarin, C.B.B.; Junior, M.R.M.; Hubinger, M.D. Anthocyanins from jussara (Euterpe eduliers Martius) extract carried by calcium alginate beads pre-prepared using ionic gelation. Powder Technol. 2019, 345, 283–291. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of cross-linking with calcium ions on the physical properties of alginate films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef]
- Liao, M.; Ma, L.; Miao, S.; Hu, X.; Liao, X.; Chen, F.; Ji, J. The in-vitro digestion behaviours of milk proteins acting as wall materials in spray-dried microparticles: Effects on the release of loaded blueberry anthocyanins. Food Hydrocoll. 2021, 115, 106620. [Google Scholar] [CrossRef]









| Beads | TAC mg C3G g−1 DW Undigested | TAC mg C3G g−1 Gastric Digestion | Recovery % | TAC mg C3G g−1 Intestinal Digestion | Bioaccessibility % |
|---|---|---|---|---|---|
| Alginate | 32.71 a ± 0.57 | 27.30 b ± 0.85 | 83.46 | 22.38 c± 0.29 | 66.41 |
| Alginate + pectin | 37.22 a ± 0.31 | 33.29 b ± 0.20 | 89.44 | 27.92 c ± 0.89 | 75 |
| Alginate + xanthan gum | 36.55 a ± 0.29 | 30.19 b ± 0.89 | 82.59 | 24.18 c ± 0.32 | 68 |
| Alginate + psyllium gum | 40.14 a ± 0.18 | 37.29 b ± 0.37 | 92.89 | 34.29 c ± 0.76 | 85.42 |
| Undigested Beads | Gastric Phase | Intestinal Phase | ||||
|---|---|---|---|---|---|---|
| L* | a* | L* | a* | L* | a* | |
| Alginate | 2.47 a ± 0.37 | 12.36 d ± 0.23 | 4.59 a ± 0.19 | 8.76 c ± 0.37 | 16.41 a ± 0.75 | 1.69 c ± 0.28 |
| Alginate + pectin | 1.63 b ± 0.17 | 13.28 c ± 0.55 | 2.67 b ± 0.26 | 12.10 b ± 0.01 | 12.65 b ± 0.83 | 2.21 b ± 0.14 |
| Alginate + xanthan gum | 1.53 b ± 0.38 | 13.94 b ± 0.79 | 2.64 b ± 0.09 | 12.64 b ± 0.13 | 12.79 b ± 0.27 | 2.11 b ± 0.79 |
| Alginate + psyllium gum | 1.36 c ± 0.27 | 16.38 a ± 0.35 | 2.59 c ± 0.19 | 14.27 a ± 0.03 | 10.55 c ± 0.13 | 3.83 a ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seke, F.; Manhivi, V.E.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. In Vitro Release of Anthocyanins from Microencapsulated Natal Plum (Carissa macrocarpa) Phenolic Extract in Alginate/Psyllium Mucilage Beads. Foods 2022, 11, 2550. https://doi.org/10.3390/foods11172550
Seke F, Manhivi VE, Slabbert RM, Sultanbawa Y, Sivakumar D. In Vitro Release of Anthocyanins from Microencapsulated Natal Plum (Carissa macrocarpa) Phenolic Extract in Alginate/Psyllium Mucilage Beads. Foods. 2022; 11(17):2550. https://doi.org/10.3390/foods11172550
Chicago/Turabian StyleSeke, Faith, Vimbainashe E. Manhivi, Retha M. Slabbert, Yasmina Sultanbawa, and Dharini Sivakumar. 2022. "In Vitro Release of Anthocyanins from Microencapsulated Natal Plum (Carissa macrocarpa) Phenolic Extract in Alginate/Psyllium Mucilage Beads" Foods 11, no. 17: 2550. https://doi.org/10.3390/foods11172550
APA StyleSeke, F., Manhivi, V. E., Slabbert, R. M., Sultanbawa, Y., & Sivakumar, D. (2022). In Vitro Release of Anthocyanins from Microencapsulated Natal Plum (Carissa macrocarpa) Phenolic Extract in Alginate/Psyllium Mucilage Beads. Foods, 11(17), 2550. https://doi.org/10.3390/foods11172550