Recent Advances and Applications in Starch for Intelligent Active Food Packaging: A Review
Abstract
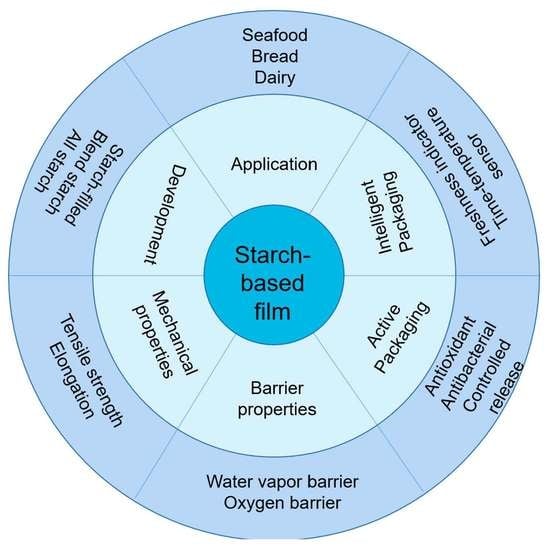
:1. Introduction
2. Starch-Based Biodegradable Film Materials
- Starch-filled plastics are made by mixing a small amount of original starch or modified starch with polyethylene or other thermoplastics and adding other applicable additives. Its purpose is to enhance the biodegradability of traditional petroleum-based starch materials. Nonetheless, its degradation still needs several years and cannot be thoroughly degraded [46];
- Blended starch plastics are made of starch mixed with synthetic resin or other natural polymer materials. They are generally blends of starch/modified starch (30–60%) and synthetic biodegradable materials, which can be completely biodegradable and do not pollute the environment [47]. Compared with purely synthetic polymers, the blends degrade quickly and have better mechanical properties. Nonetheless, the added synthetic resins or other natural polymer materials are primarily polar compounds with hydrophilicity, and long-term exposure or contact with water will considerably degrade the properties of the plastic [48]. In addition, the compatibility between starch and additives, such as synthetic resins or other natural polymers is likewise problematic [49];
- All starch plastics, also known as thermoplastic starch plastic, are a natural polymer biodegradable material. They are prepared by adding degradable plasticizers and other additives [50] through processes, such as extrusion, injection moulding, blow moulding, and calendering, which result in a “disordered” arrangement of starch molecule. The starch content of all starch plastics is above 90%, and a small number of different substances added as additives are nontoxic and can be completely degraded. Thus, all starchy plastics are genuinely and completely biodegradable. In addition, almost all plastic processing methods can be applied to all starch plastics [51].
3. Mechanical Properties of Starch-Based Films
4. Hydrophobic and Barrier Properties of Starch-Based Films
4.1. Water Vapor Barrier
4.2. Oxygen Barrier
5. Starch-Based Active Films
5.1. Antioxidant Active Starch-Based Films
5.2. Antibacterial Active Starch-Based Films
5.3. Controlled Release Starch-Based Active Films
6. Starch-Based Intelligent Films
6.1. Freshness Indicator
6.2. Time-Temperature Sensor
7. Starch-Based Active and Intelligent Films Application in the Food Industry
7.1. Active Packaging
7.2. Intelligent Packaging
8. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PB | Pinto Bean Starch |
| PV | Polyvinyl Alcohol |
| NF | native starch |
| PSE | pecan nutshell extract |
| HSE | hazelnut skin extract |
| WVP | water vapor permeability |
| CR | curcumin |
| ATH | anthocyanin |
References
- Koketso, N.L.; Uchenna, U.A.; Nifise, O.E.; Rozli, Z.; Nongwe, B.I. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar]
- Kumar, G.M.; Irshad, A.; Raghunath, B.; Rajarajan, G. Waste management in food packaging industry. In Integrated Waste Management in India; Springer: Heidelberg/Berlin, Germany, 2016; pp. 265–277. [Google Scholar]
- Ellen, P.; Jacob, H.; Karl, H.; Dan, N.T. Narrating plastics governance: Policy narratives in the European plastics strategy. Environ. Politics 2022, 31, 365–385. [Google Scholar]
- Biron, M. Thermoplastics and Thermoplastic Composites; William Andrew: Norwich, NY, USA, 2018. [Google Scholar]
- Williams, K.S. Plastic Packaging: Not a Throw-away Resource. In Issues in Environmental Science and Technology: Waste as a Resource; The Royal Society for Chemistry: Cambridge, UK, 2013; pp. 83–109. [Google Scholar]
- Robertson, G.L. Definitions, Functions, Attributes and Environments of Food Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263 Pt A, 114469. [Google Scholar] [CrossRef]
- Lokesh, K.; Dakuri, R.; Konala, A.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2021, 52, 533–552. [Google Scholar]
- Moshood, T.D.; Gusman, N.; Fatimah, M.; Fazeeda, M.; Hanafiah, A.M.; Airin, A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Fan, Z. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar]
- Anna, K.; Katarzyna, K.; Katarzyna, P.; Mariola, S.; Ewa, S.; Paulina, H. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar]
- Sergio, J.C.-E.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Protein-Based Films: Advances in the Development of Biomaterials Applicable to Food Packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar]
- Yunpeng, X.; Ying, W.; Tao, Z.; Guangqing, M.; Shujuan, J.; Xuemei, Z.; Yanfeng, T.; Fang, Q. Evaluation of the properties of whey protein films with modifications. J. Food Sci. 2021, 86, 923–931. [Google Scholar]
- Ochoa, T.A.; Almendárez, B.E.G.; Reyes, A.A.; Dulce, M.; Rivera, P.; Gustavo, F.; Gutiérrez, L.; Olga Martín, B.; Carlos, R.-G. Design and Characterization of Corn Starch Edible Films Including Beeswax and Natural Antimicrobials. Food Bioprocess Technol. 2017, 10, 103–114. [Google Scholar] [CrossRef]
- Usman, A.; Usman, K.M.; Yaqoob, M.; Maksim, R.; Mars, K.; Elena, B.; Ali, S.M.; Min, C.I.; Muthu, T. Potentials of polysaccharides, lipids and proteins in biodegradable food packaging applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar]
- Shilpi, A. Major factors affecting the characteristics of starch based biopolymer films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar]
- Guo, B.; Wang, Y.; Pang, M.; Wu, J.; Hu, X.; Huang, Z.; Wang, H.; Xu, S.; Luo, S.; Liu, C. Annealing treatment of amylose and amylopectin extracted from rice starch. Int. J. Biol. Macromol. 2020, 164, 3496–3500. [Google Scholar] [CrossRef]
- Ettelaie, R.; Holmes, M.; Chen, J.; Farshchi, A. Steric stabilising properties of hydrophobically modified starch: Amylose vs. amylopectin. Food Hydrocoll. 2016, 58, 364–377. [Google Scholar] [CrossRef]
- Punia, B.S.; Omodunbi, A.A.; Arashdeep, S.; Vandana, C.; Scott, W.W. Enzymatic modification of starch: A green approach for starch applications. Carbohydr. Polym. 2022, 287, 119265. [Google Scholar] [CrossRef]
- Khan, B.; Niazi, M.B.K.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material—A Review. J. Food Process Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Shang, X.; Xie, F. Starch–zinc complex and its reinforcement effect on starch-based materials. Carbohydr. Polym. 2018, 206, 528–538. [Google Scholar] [CrossRef]
- Congli, C.; Na, J.; Yanfei, W.; Liu, X.; Qingjie, S. Bioactive and intelligent starch-based films: A review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar]
- Weerapoprasit, C.; Prachayawarakorn, J. Effects of Polymethacrylamide-Grafted Branch on Mechanical Performances, Hydrophilicity, and Biodegradability of Thermoplastic Starch Film. Starch-Stärke 2019, 71, 11–12. [Google Scholar] [CrossRef]
- Kwaśniewska, A.; Chocyk, D.; Gładyszewski, G.; Borc, J.; Świetlicki, M.; Gładyszewska, B. The Influence of Kaolin Clay on the Mechanical Properties and Structure of Thermoplastic Starch Films. Polymers 2020, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, S.M.; Maryam, A.; Milad, T.; Keyhan, M.; Julian, M.D. Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials. Nanomaterials 2021, 11, 1331. [Google Scholar]
- Vilas, C.; Mauricio-Iglesias, M.; García, M.R. Model-based design of smart active packaging systems with antimicrobial activity. Food Packag. Shelf Life 2020, 24, 100446. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar]
- Tarsila, R.A.; Patrícia, C.B.; Allan, R.F.e.M.; Nilda, d.F.F.S. Natural bioactives in perspective: The future of active packaging based on essential oils and plant extracts themselves and those complexed by cyclodextrins. Food Res. Int. 2022, 156, 111160. [Google Scholar]
- Menzel, C.; González-Martínez, C.; Vilaplana, F.; Diretto, G.; Chiralt, A. Incorporation of natural antioxidants from rice straw into renewable starch films. Int. J. Biol. Macromol. 2020, 146, 976–986. [Google Scholar] [CrossRef]
- Meng, C.; Yingjun, C.; Xiaoran, Y.; Rongfei, Z.; Juan, W.; Xiangyou, W. Effect of dual-modified cassava starches on intelligent packaging films containing red cabbage extracts. Food Hydrocoll. 2022, 124, 107225. [Google Scholar]
- Luman, Z.; Liming, L.; Jiahao, Y.; Ping, S. Novel trends and applications of natural pH-responsive indicator film in food packaging for improved quality monitoring. Food Control 2022, 134, 108769. [Google Scholar]
- Helen, O.; KeChrist, O.; Golden, M.; Nwabunwanne, N. Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging. Polymers 2022, 14, 1126. [Google Scholar]
- Liu, W.; Wang, Z.; Liu, J.; Dai, B.; Hu, S.; Hong, R.; Xie, H.; Li, Z.; Chen, Y.; Zeng, G. Preparation, reinforcement and properties of thermoplastic starch film by film blowing. Food Hydrocoll. 2020, 108, 106006. [Google Scholar] [CrossRef]
- Larissa, d.V.S.; la Fuente, A.C.I.; Chieregato, M.B.; Cecília, T.C. Starch-based biodegradable plastics: Methods of production, challenges and future perspectives. Curr. Opin. Food Sci. 2020, 38, 122–130. [Google Scholar]
- Chuanyan, G.; Hongge, G. Progress in the Degradability of Biodegradable Film Materials for Packaging. Membranes 2022, 12, 500. [Google Scholar]
- Hao, C.; Long, C.; Julian, M.D.; Tianyi, Y.; Zipei, Z.; Fei, R.; Ming, M.; Yaoqi, T.; Zhengyu, J. Starch-based biodegradable packaging materials: A review of their preparation, characterization and diverse applications in the food industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- da Silva, L.R.; de Carvalho, C.W.P.; Velasco, J.I.; Fakhouri, F.M. Extraction and characterization of starches from pigmented rice. Int. J. Biol. Macromol. 2020, 156, 485–493. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Adeloye, A.A.; Olaomo, O.O.; Kayitesi, E. Effect of fermentation time on physicochemical properties of starch extracted from cassava root. Food Biosci. 2020, 33, 100485. [Google Scholar] [CrossRef]
- Madsar, H.; Ahmad, S.W.; Sajjad, A.; Qutab, H.G.; Muhammad, D.; Muhammad, I. Enzymatic extraction of potato starch: A parametric optimization study using response surface methodology. Pol. J. Chem. Technol. 2020, 22, 48–54. [Google Scholar]
- Hernández-Carmona, F.; Morales-Matos, Y.; Lambis-Miranda, H.; Pasqualino, J. Starch extraction potential from plantain peel wastes. J. Environ. Chem. Eng. 2017, 5, 4980–4985. [Google Scholar] [CrossRef]
- Lu, Z.; Donner, E.; Liu, Q. The Effect of Various Extracting Agents on the Physicochemical and Nutritional Properties of Pea Starch. Starch-Stärke 2019, 71, 11–12. [Google Scholar] [CrossRef]
- Kringel, D.H.; Dias, A.R.G.; Zavareze, E.d.; Gandra, E.A. Fruit Wastes as Promising Sources of Starch: Extraction, Properties, and Applications. Starch-Stärke 2020, 72, 3–4. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.; Song, K.B. Development of a Sword Bean (Canavalia gladiata) Starch Film Containing Goji Berry Extract. Food Bioprocess Technol. 2020, 13, 911–921. [Google Scholar] [CrossRef]
- Zuzanna, Ż.; Alicja, K. The Influence of Starch Origin on the Properties of Starch Films: Packaging Performance. Materials 2021, 14, 1146. [Google Scholar]
- Abioye, A.A.; Obuekwe, C.C. Investigation of the biodegradation of low-density polyethylene-starch Bi-polymer blends. Results Eng. 2020, 5, 100090. [Google Scholar] [CrossRef]
- Dammak, M.; Fourati, Y.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Blends of PBAT with plasticized starch for packaging applications: Mechanical properties, rheological behaviour and biodegradability. Ind. Crops Prod. 2020, 144, 112061. [Google Scholar] [CrossRef]
- Castaño, J.; Rodríguez-Llamazares, S.; Sepúlveda, E.; Giraldo, D.; Bouza, R.; Pozo, C. Morphological and structural changes of starch during processing by melt blending. Starch-Stärke 2017, 69, 9–10. [Google Scholar] [CrossRef]
- Huan, H.; Ang, X.; Dianfeng, Z.; Weiyi, Z.; Shaoxian, P.; Xipo, Z. High-Toughness Poly(Lactic Acid)/Starch Blends Prepared through Reactive Blending Plasticization and Compatibilization. Molecules 2020, 25, 5951. [Google Scholar]
- Hirpara, N.J.; Dabhi, M.N. Development of Potato Starch Based Biodegradable Packaging Film. J. Food Process. Technol. 2021, 12, 529–541. [Google Scholar]
- Gülay, B.; Faik, D. Investigation and preparation of biodegradable starch-based nanofilms for potential use of curcumin and garlic in food packaging applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 1127–1143. [Google Scholar]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Kuz, P.; Ateş, M. Starch-Based Bioplastic Materials for Packaging Industry. J. Sustain. Constr. Mater. Technol. 2020, 5, 399–406. [Google Scholar]
- Lauer, M.K.; Smith, R.C. Recent advances in starch-based films toward food packaging applications: Physicochemical, mechanical, and functional properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083. [Google Scholar] [CrossRef]
- Vu, H.P.N.; Lumdubwong, N. Starch behaviors and mechanical properties of starch blend films with different plasticizers. Carbohydr. Polym. 2016, 154, 112–120. [Google Scholar]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Ali, K. Evaluation of Physical, Mechanical and Antibacterial Properties of Pinto Bean Starch-Polyvinyl Alcohol Biodegradable Films Reinforced with Cinnamon Essential Oil. Polymers 2021, 13, 2778. [Google Scholar]
- Shaikh, M.; Haider, S.; Ali, T.M.; Hasnain, A. Physical, thermal, mechanical and barrier properties of pearl millet starch films as affected by levels of acetylation and hydroxypropylation. Int. J. Biol. Macromol. 2018, 124, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Namory, M.; Koffi, K.L.; Tohoué, T.M.; Samuel, O. Effect of metakaolin content on mechanical and water barrier properties of cassava starch films. S. Afr. J. Chem. Eng. 2022, 40, s186–s194. [Google Scholar]
- Pornchai, R.; Sarinthip, T.; Auras, A.R.; Nareekan, C.; Kittisak, J.; Pensak, J.; Yuthana, P.; Phisit, S.; Noppol, L.; Thanongsak, C.; et al. Morphology, Mechanical, and Water Barrier Properties of Carboxymethyl Rice Starch Films: Sodium Hydroxide Effect. Molecules 2022, 27, 331. [Google Scholar]
- Leon-Bejarano, M.; Durmus, Y.; Ovando-Martínez, M.; Simsek, S. Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut By-Products Extracts. Foods 2020, 9, 226. [Google Scholar] [CrossRef]
- Cano, A.; Jiménez, A.; Cháfer, M.; Gónzalez, C.; Chiralt, A. Effect of amylose:amylopectin ratio and rice bran addition on starch films properties. Carbohydr. Polym. 2014, 111, 543–555. [Google Scholar] [CrossRef]
- Yuyue, Z.; Lingyu, T.; Andreas, B.; Li, D.; Klaus, H.; Jianzhou, Q.; Anzhou, X.; Dongwei, G.; Henrik, H.K.; Xingxun, L. High-amylose starch: Structure, functionality and applications. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Vu, H.P.N.; Lumdubwong, N. Fabrication of starch blend films with different matrices and their mechanical properties. Polym. Test. 2020, 90, 106694. [Google Scholar]
- Pająk, P.; Przetaczek-Rożnowska, I.; Juszczak, L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. Int. J. Biol. Macromol. 2019, 138, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Totosaus, A.; Godoy, I.A.; Ariza, O.T.J. Structural and mechanical properties of edible films from composite mixtures of starch, dextrin and different types of chemically modified starch. Int. J. Polym. Anal. Charact. 2020, 25, 517–528. [Google Scholar] [CrossRef]
- Peng, Y.; Chunhao, C.; Hongpeng, M.; Huijuan, G.; Bin, G.; Panxin, L. Surface cross-linked thermoplastic starch with different UV wavelengths: Mechanical, wettability, hygroscopic and degradation properties. RSC Adv. 2020, 10, 44815–44823. [Google Scholar]
- Dai, L.; Zhang, J.; Cheng, F. Effects of starches from different botanical sources and modification methods on physicochemical properties of starch-based edible films. Int. J. Biol. Macromol. 2019, 132, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.d.A.; Menegalli, F.C. Nanocomposites based on banana starch reinforced with cellulose nanofibers isolated from banana peels. J. Colloid Interface Sci. 2017, 505, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, C.; Wang, X.; Xiong, L.; Sun, Q. Physicochemical properties of starch nanocomposite films enhanced by self-assembled potato starch nanoparticles. LWT-Food Sci. Technol. 2016, 69, 251–257. [Google Scholar] [CrossRef]
- Travalini, A.P.; Lamsal, B.; Magalhães, W.L.E.; Demiate, I.M. Cassava starch films reinforced with lignocellulose nanofibers from cassava bagasse. Int. J. Biol. Macromol. 2019, 139, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Antonia, A.; Rolf, I.; Verena, R.; Werner, R.; Dietrich, H.; Judith, K. Implementation of Time Temperature Indicators to Improve Temperature Monitoring and Support Dynamic Shelf Life in Meat Supply Chains. J. Packag. Technol. Res. 2020, 4, 23–32. [Google Scholar]
- van Soest, J.J.G.; Hulleman, S.H.D.; de Wit, D.; Vliegenthart, J.F.G. Changes in the mechanical properties of thermoplastic potato starch in relation with changes in Btype crystallinity. Carbohydr. Polym. 1996, 29, 225–232. [Google Scholar] [CrossRef]
- Ren, L.; Fu, Y.; Chang, Y.; Jiang, M.; Tong, J.; Zhou, J. Performance improvement of starch films reinforced with starch nanocrystals (SNCs) modified by cross-linking. Starch-Stärke 2017, 69. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Amylose-lipid complex as a measure of variations in physical, mechanical and barrier attributes of rice starch- ι -carrageenan biodegradable edible film. Food Packag. Shelf Life 2017, 14, 108–115. [Google Scholar] [CrossRef]
- Ni, S.; Zhang, H.; Dai, H.; Xiao, H. Starch-Based Flexible Coating for Food Packaging Paper with Exceptional Hydrophobicity and Antimicrobial Activity. Polymers 2018, 10, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Huang, L.; Zhang, C.; Deng, Y.; Xie, P.; Liu, L.; Cheng, J. Research advances in chemical modifications of starch for hydrophobicity and its applications: A review. Carbohydr. Polym. 2020, 240, 116292. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Perdomo, J.; Cova, A.; Sandoval, A.J.; García, L.; Laredo, E.; Müller, A.J. Glass transition temperatures and water sorption isotherms of cassava starch. Carbohydr. Polym. 2009, 76, 305–313. [Google Scholar] [CrossRef]
- Chenglong, L.; Bin, Y.; Haiteng, T.; Pengfei, L.; Haibo, Z.; Congping, T.; Bo, C. Effects of soy protein isolate on mechanical and hydrophobic properties of oxidized corn starch film. LWT 2021, 147, 111529. [Google Scholar]
- Frida, I.; Krister, H.; Romain, B. Surface Treatment by Hydrophobic Particles: Influence of Starch and Ionic Strength. ACS Sustain. Chem. Eng. 2017, 5, 6107–6115. [Google Scholar]
- Phanwipa, W.; Theeraphorn, P.; Nathdanai, H. Effect of different modified starches on physical, morphological, thermomechanical, barrier and biodegradation properties of cassava starch and polybutylene adipate terephthalate blend film. Food Packag. Shelf Life 2022, 32, 100844. [Google Scholar]
- Milani, M.J.; Nemati, A. Lipid-Based Edible Films and Coatings: A Review of Recent Advances and Applications. J. Packag. Technol. Res. 2022, 6, 11–22. [Google Scholar] [CrossRef]
- Bourtoom, T. Improvement of Water Barrier Property of Rice Starch-chitosan Composite Film Incorporated with Lipids. Food Sci. Technol. Int. 2009, 15, 149–158. [Google Scholar] [CrossRef]
- Tavares, K.M.; de Campos, A.; Mitsuyuki, M.C.; Luchesi, B.R.; Marconcini, J.M. Corn and cassava starch with carboxymethyl cellulose films and its mechanical and hydrophobic properties. Carbohydr. Polym. 2019, 223, 115055. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.L.; Wu, J.M.; Chen, Y.; Zhao, G. Antimicrobial and physical properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocoll. 2009, 24, 285.0–290.0. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Cheng, M. Preparation and Characterization of Potato Starch Film with Various Size of Nano-SiO2. Polymers 2018, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Zhang, H.; Godwin, P.M.; Dai, H.; Xiao, H. ZnO nanoparticles enhanced hydrophobicity for starch film and paper. Mater. Lett. 2018, 230, 207–210. [Google Scholar] [CrossRef]
- Onwulata, C.I. Food Packaging Principles and Practice, 3rd ed.; Robertson, G.L., Ed.; CRC Press Taylor & Francis Group: Boca Rotan, FL, USA; p. 2055.
- Wattinee, K.; Phanwipa, W.; Phatthranit, K.; Nathdanai, H. Thermoplastic starch blown films functionalized by plasticized nitrite blended with PBAT for superior oxygen barrier and active biodegradable meat packaging. Food Chem. 2022, 374, 131709. [Google Scholar]
- Sethy, P.K.; Prusty, K.; Mohapatra, P.; Swain, S.K. Nanoclay decorated polyacrylic acid/starch hybrid nanocomposite thin films as packaging materials. Polym. Compos. 2019, 40, 229–239. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Jia, R.; Dai, Y.; Dong, H.; Hou, H.; Guo, Q. High performance extrusion blown starch/polyvinyl alcohol/clay nanocomposite films. Food Hydrocoll. 2018, 79, 534–543. [Google Scholar] [CrossRef]
- Salman, K.; Saqib, A.; Mohsin, A.T.; Mehwish, I.H.; Marium, S.; Haris, K.; QurratulAin, A.; Shahid, Y. Effect of Silver Nanoparticles Prepared from Saraca asoca Leaf Extract on Morphological, Functional, Mechanical, and Antibacterial Properties of Rice Starch Films. Starch-Stärke 2022, 74, 2100228. [Google Scholar]
- Yue, C.; Shan, G.; Wen-tao, W.; Han-xue, H.; Loong-Tak, L. Low temperature extrusion blown ε-polylysine hydrochloride-loaded starch/gelatin edible antimicrobial films. Carbohydr. Polym. 2022, 278, 118990. [Google Scholar]
- Nattinee, B.; Jungwook, C.; Seonghyuk, K. Applications of Nanomaterials in Food Packaging. J. Nanosci. Nanotechnol. 2015, 15, 6357–6372. [Google Scholar]
- Zheng, K.; Li, W.; Fu, B.; Fu, M.; Ren, Q.; Yang, F.; Qin, C. Physical, antibacterial and antioxidant properties of chitosan films containing hardleaf oatchestnut starch and Litsea cubeba oil. Int. J. Biol. Macromol. 2018, 118, 707–715. [Google Scholar] [CrossRef]
- Rungsima, C.; Siraprapa, P.; Watthana, B.; Nattaporn, K.; Kunat, K.; Rungsinee, S.; Prakit, S.; Udomlak, S.; Nathdanai, H. Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. LWT 2020, 130, 109573. [Google Scholar]
- Pardeep, K.; Rohit, T.; Vidhi, G.; Aakash, U.; Anil, K.; Kirtiraj, G.K. Pineapple peel extract incorporated poly(vinyl alcohol)-corn starch film for active food packaging: Preparation, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar]
- Gaikwad, K.K.; Suman, S.; Suk, L.Y. A new pyrogallol coated oxygen scavenging film and their effect on oxidative stability of soybean oil under different storage conditions. Food Sci. Biotechnol. 2017, 26, 1535–1543. [Google Scholar] [CrossRef]
- Nisa, I.U.; Ashwar, B.A.; Shah, A.; Gani, A.; Gani, A.; Masoodi, F.A. Development of potato starch based active packaging films loaded with antioxidants and its effect on shelf life of beef. J. Food Sci. Technol. 2015, 52, 7245–7253. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Aurrekoetxea, G.P.; Angulo, I.; Paseiro-Losada, P.; Cruz, J.M. Development of new active packaging films coated with natural phenolic compounds to improve the oxidative stability of beef. Meat Sci. 2014, 97, 249–254. [Google Scholar] [CrossRef]
- Cláudia, S.A.A.; Francielly, C.A.; Maria, d.S.F.e.S.L.; Aparecida, C.F.L.; Emília, d.S.G.M.; José, P.C. Effect of natural and synthetic antioxidants on oxidation and storage stability of mechanically separated tilapia meat. LWT 2022, 154, 112679. [Google Scholar]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Biodegradability and disintegration of multilayer starch films with electrospun PCL fibres encapsulating carvacrol. Polym. Degrad. Stab. 2020, 173, 109100. [Google Scholar] [CrossRef]
- Kumar, J.; Akhila, K.; Gaikwad, K.K. Recent Developments in Intelligent Packaging Systems for Food Processing Industry: A Review. J. Food Process. Technol. 2021, 12, 895. [Google Scholar]
- Kowalczyk, D.; Kazimierczak, W.; Zięba, E.; Mężyńska, M.; Basiura-Cembala, M.; Lisiecki, S.; Karaś, M.; Baraniak, B. Ascorbic acid- and sodium ascorbate-loaded oxidized potato starch films: Comparative evaluation of physicochemical and antioxidant properties. Carbohydr. Polym. 2018, 181, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Amiri, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R. Combined antioxidant and sensory effects of corn starch films with nanoemulsion of Zataria multiflora essential oil fortified with cinnamaldehyde on fresh ground beef patties. Meat Sci. 2019, 153, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ângelo, L.; Ana, R.; Fernanda, D. Pullulan Films Containing Rockrose Essential Oil for Potential Food Packaging Applications. Antibiotics 2020, 9, 681. [Google Scholar]
- William, O.; Zhong, Z.; Yang, B.; Reza, T. Application of starch-based coatings incorporated with antimicrobial agents for preservation of fruits and vegetables: A review. Prog. Org. Coat. 2022, 166, 106800. [Google Scholar]
- Muller, J.; Quesada, A.C.; González-Martínez, C.; Chiralt, A. Antimicrobial properties and release of cinnamaldehyde in bilayer films based on polylactic acid (PLA) and starch. Eur. Polym. J. 2017, 96, 316–325. [Google Scholar] [CrossRef]
- Anwar, M.; Istiqomah, L.; Ekaningrum, M.; Yembise, D. Antibacterial activities of biocomposite plastic-based phenolic acids-grafted chitosan and sugar palm starch (Arenga pinata). IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 012046. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Characterization of pea starch-guar gum biocomposite edible films enriched by natural antimicrobial agents for active food packaging. Food Bioprod. Process. 2017, 105, 51–63. [Google Scholar] [CrossRef]
- Evangelho, J.A.d.; Dannenberg, G.d.; Biduski, B.; el Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; Zavareze, E.D. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef]
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Basha, R.K.; Naim, M.N. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef]
- Martins, P.C.; Bagatini, D.C.; Martins, V.G. Oregano essential oil addition in rice starch films and its effects on the chilled fish storage. J. Food Sci. Technol. 2020, 58, 1562–1573. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Atarés, L.; Vargas, M.; Chiralt, A. Physical and Antimicrobial Properties of Compression-Molded Cassava Starch-Chitosan Films for Meat Preservation. Food Bioprocess Technol. 2018, 11, 1339–1349. [Google Scholar] [CrossRef]
- Yasa, S.R.; Kaki, S.S.; Poornachandra, Y.; Kumar, C.G.; Penumarthy, V. Synthesis, characterization, antimicrobial and biofilm inhibitory studies of new esterquats. Bioorganic Med. Chem. Lett. 2016, 26, 1978–1982. [Google Scholar] [CrossRef] [PubMed]
- Zexin, W. Talking about the New Uses of Several Inorganic Antibacterial Materials. J. Phys. Conf. Ser. 2021, 1965, 012076. [Google Scholar]
- Chen, R.; Han, Z.; Huang, Z.; Karki, J.; Wang, C.; Zhu, B.; Zhang, X. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mater. J. 2017, 36, 693–699. [Google Scholar] [CrossRef]
- Hu, X.; Jia, X.; Zhi, C.; Jin, Z.; Miao, M. Improving the properties of starch-based antimicrobial composite films using ZnO-chitosan nanoparticles. Carbohydr. Polym. 2019, 210, 204–209. [Google Scholar] [CrossRef]
- Chenwei, C.; Chenxi, L.; Shaohua, Y.; Qinjun, Z.; Fuxin, Y.; Zhipeng, T.; Jing, X. Development of New Multilayer Active Packaging Films with Controlled Release Property Based on Polypropylene/Poly(Vinyl Alcohol)/Polypropylene Incorporated with Tea Polyphenols. J. Food Sci. 2019, 84, 1836–1843. [Google Scholar] [CrossRef]
- Chenwei, C.; Zhewei, X.; Yarui, M.; Jinliang, L.; Qinjun, Z.; Zhipeng, T.; Kaijia, F.; Fuxin, Y.; Jing, X. Properties, vapour-phase antimicrobial and antioxidant activities of active poly(vinyl alcohol) packaging films incorporated with clove oil. Food Control 2018, 88, 105–112. [Google Scholar] [CrossRef]
- Hadi, A.; Mahsa, J.O.; Ayda, S. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–21. [Google Scholar]
- Cornelia, V.; Mihaela, B. Progresses in Food Packaging, Food Quality, and Safety—Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796.0–812.0. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Mastromatteo, M.; Conte, A.; del Nobile, M.A. Advances in controlled release devices for food packaging applications. Trends Food Sci. Technol. 2010, 21, 591–598. [Google Scholar] [CrossRef]
- Fernando, M.Y.D.; Walter, M.A.; Arturo, J.R.Á.; Jairo, M.A.J.; Esteban, A.P.Á. Encapsulation of phenols of gulupa seed extract using acylated rice starch: Effect on the release and antioxidant activity. J. Funct. Foods 2021, 87, 104788. [Google Scholar]
- Zhang, S.; Zhao, H. Preparation and properties of zein–rutin composite nanoparticle/corn starch films. Carbohydr. Polym. 2017, 169, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Starch films loaded with donut-shaped starch-quercetin microparticles: Characterization and release kinetics. Int. J. Biol. Macromol. 2018, 118, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Sajad, P.; Karimi, S.I.; Sadat, M.S. Nano-biocomposite based color sensors: Investigation of structure, function, and applications in intelligent food packaging. Food Packag. Shelf Life 2022, 31, 100789. [Google Scholar]
- Chen, W.; Ma, S.; Wang, Q.; Julian, M.D.; Liu, X.; Ngai, T.; Liu, F. Fortification of edible films with bioactive agents: A review of their formation, properties, and application in food preservation. Crit. Rev. Food Sci. Nutr. 2021, 62, 5029–5055. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT-Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Nitya, B.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar]
- Swarup, R.; Jong-Whan, R. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–29. [Google Scholar]
- Golasz, L.B.; da Silva, J.; da Silva, S.B. Film with anthocyanins as an indicator of chilled pork deterioration. Food Sci. Technol. 2013, 33, 155–162. [Google Scholar] [CrossRef]
- Etxabide, A.; Kilmartin, P.A.; Maté, J.I. Color stability and pH-indicator ability of curcumin, anthocyanin and betanin containing colorants under different storage conditions for intelligent packaging development. Food Control 2021, 121, 107645. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Anabel, V.; Alfredo, S.; Beatriz, M.; Julio, R.; Reynaldo, V. Electrochemical biosensors for food bioprocess monitoring. Curr. Opin. Food Sci. 2022, 43, 18–26. [Google Scholar]
- Boyu, M.; Guoqing, C.; Luwei, Z.; Yu, Z.; Xinqing, X. Flexible wireless pH sensor system for fish monitoring. Sens. Bio-Sens. Res. 2021, 34, 100465. [Google Scholar]
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of Time Temperature Indicators as Quality Monitors in Food Packaging. Packag. Technol. Sci. 2015, 28, 839.0–867.0. [Google Scholar] [CrossRef]
- Hao, C.; Hao, X.; David, J.M.; Long, C.; Aiquan, J.; Yaoqi, T.; Ming, M.; Zhengyu, J. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2021, 375, 131738. [Google Scholar]
- Zhang, X.; Sun, G.; Xiao, X.; Liu, Y.; Zheng, X. Application of microbial TTIs as smart label for food quality: Response mechanism, application and research trends. Trends Food Sci. Technol. 2016, 51, 12–23. [Google Scholar] [CrossRef]
- Preetam, A.; Dominic, S.; Anastasia, E.L. Time-Temperature Indicator Based on Enzymatic Degradation of Dye-Loaded Polyhydroxybutyrate. Biotechnol. J. 2017, 12, 1700050. [Google Scholar]
- Jafry, A.T.; Lim, H.; Sung, W.K.; Lee, J. Flexible time–temperature indicator: A versatile platform for laminated paper-based analytical devices. Microfluid. Nanofluid 2017, 21, 57. [Google Scholar] [CrossRef]
- Kim, J.U.; Ghafoor, K.; Ahn, J.; Shin, S.; Lee, S.H.; Shahbaz, H.M.; Shin, H.; Kim, S.; Park, J. Kinetic modeling and characterization of a diffusion-based time-temperature indicator (TTI) for monitoring microbial quality of non-pasteurized angelica juice. LWT-Food Sci. Technol. 2016, 67, 143–150. [Google Scholar] [CrossRef]
- Santos, C.T.; Veiga-Santos, P.; Sestari, P.; Sorrini, N.C.; Roça, R.O. Protein time–temperature sensor for intelligent starch polymers. J. Food Process. Preserv. 2020, 44, e14428. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Incorporation of spray dried and freeze dried blackberry particles in edible films: Morphology, stability to pH, sterilization and biodegradation. Food Packag. Shelf Life 2019, 20, 100313. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An active packaging film based on yam starch with eugenol and its application for pork preservation. Food Hydrocoll. 2019, 96, 546.0–554.0. [Google Scholar] [CrossRef]
- Kang, J.; Song, K.B. Characterization of Job’s tears (Coix lachryma-jobi L.) starch films incorporated with clove bud essential oil and their antioxidant effects on pork belly during storage. LWT 2019, 111, 711–718. [Google Scholar] [CrossRef]
- Goodarzi, M.M.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Development of an easy-to-use colorimetric pH label with starch and carrot anthocyanins for milk shelf life assessment. Int. J. Biol. Macromol. 2020, 153, 240–247. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Silva, O.A.; Pellá, M.G.; Beneton, A.G.; Caetano, J.; Simões, M.R.; Dragunski, D.C. Effect of gelatin and casein additions on starch edible biodegradable films for fruit surface coating. Food Chem. 2020, 309, 125764. [Google Scholar] [CrossRef]
- Meral, Y.; Hasan, S.; Mahmut, Ş. Characterization of edible film based on grape juice and cross-linked maize starch and its effects on the storage quality of chicken breast fillets. LWT 2021, 142, 111012. [Google Scholar] [CrossRef]
- Gustavo, S.W.; Gonçalves, d.C.; Gomes, d.A.P.; Peruzzo, F.J.; Stremel, A.M.; Tânia, F.C.; Ramos, N.M.; Paula, d.V.A. Application in situ of biodegradable films produced with starch, citric pectin and functionalized with feijoa (Acca sellowiana (Berg) Burret) extracts: An effective proposal for food conservation. Int. J. Biol. Macromol. 2021, 189, 544–553. [Google Scholar]
- Ni, S.; Jiao, L.; Zhang, H.; Zhang, Y.; Fang, G.; Xiao, H.; Dai, H. Enhancing hydrophobicity, strength and UV shielding capacity of starch film via novel co-cross-linking in neutral conditions. R. Soc. Open Sci. 2018, 5, 181206. [Google Scholar]
- Shahab, N.; Masoud, R.; Mehdi, A. A starch-based pH-sensing and ammonia detector film containing betacyanin of paperflower for application in intelligent packaging of fish. Int. J. Biol. Macromol. 2021, 191, 161–170. [Google Scholar]
- Xinxin, L.; Di, L.; Shuai, D.; Ling, Z.; Kang, M. Corn starch/polyvinyl alcohol based films incorporated with curcumin-loaded Pickering emulsion for application in intelligent packaging. Int. J. Biol. Macromol. 2021, 188, 974–982. [Google Scholar]
- Yan, Q.; Dawei, Y.; Fengfeng, X.; Dan, C.; Juan, K.; Jun, L. Smart packaging films based on starch/polyvinyl alcohol and Lycium ruthenicum anthocyanins-loaded nano-complexes: Functionality, stability and application. Food Hydrocoll. 2021, 119, 106850. [Google Scholar]
- Goudar, H.V.D.; Gasti, N.; Khanapure, T.; Vanjeri, S.; Sataraddi, V.N.; D’souza, S.; Oshin, V.J.; Kumar, S.; Saraswati, M.P.; Malabadi, R.B.; et al. Exploration of Multifunctional Properties of Piper betel Leaves Extract Incorporated Polyvinyl Alcohol-Oxidized Maize Starch Blend Films for Active Packaging Applications. J. Polym. Environ. 2022, 30, 1314–1329. [Google Scholar]
- Zhikun, M.; Yanfei, Z.; Panfang, L. Novel active starch films incorporating tea polyphenols-loaded porous starch as food packaging materials. Int. J. Biol. Macromol. 2021, 192, 1123–1133. [Google Scholar]
- Shi, C.; Zhang, X.; Guo, N. The antimicrobial activities and action-mechanism of tea tree oil against food-borne bacteria in fresh cucumber juice. Microb. Pathog. 2018, 125, 262–271. [Google Scholar] [CrossRef]
- Quintavalla, S.; Vicini, L. Antimicrobial food packaging in meat industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Sanfuentes, E.A. The synergistic antimicrobial effect of carvacrol and thymol in clay/polymer nanocomposite films over strawberry gray mold. LWT-Food Sci. Technol. 2015, 64, 390–396. [Google Scholar] [CrossRef]
- Bin, L.; Yiwen, B.; Jiaxin, L.; Jinfeng, B.; Qinqin, C.; Huijun, C.; Yuxuan, W.; Jinlong, T.; Chi, S.; Yuehua, W.; et al. A sub-freshness monitoring chitosan/starch-based colorimetric film for improving color recognition accuracy via controlling the pH value of the film-forming solution. Food Chem. 2022, 388, 132975. [Google Scholar]
- Chen, H.; Zhang, M.; Bhandari, B.; Yang, C. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almasi, H.; Moradi, M. Immobilization of Echium amoenum anthocyanins into bacterial cellulose film: A novel colorimetric pH indicator for freshness/spoilage monitoring of shrimp. Food Control 2020, 113, 107169. [Google Scholar] [CrossRef]



| Films | Additives | Thickness (mm) | Moisture Content (%) | Tensile Strength (MPa) | Elongation(%) | References |
|---|---|---|---|---|---|---|
| Cassava starch Mungbean starch Cassava: Mungbean (50:50) | glycerol | 0.103 | 19.22 | 2.85 | 18.82 | [55] |
| 0.098 | 19.66 | 9.34 | 21.37 | |||
| 0.090 | 22.11 | 7.93 | 21.32 | |||
| sorbitol | 0.101 | 9.43 | 6.77 | 14.86 | ||
| 0.113 | 9.16 | 19.20 | 12.89 | |||
| 0.105 | 8.84 | 15.87 | 10.84 | |||
| Wheat | glycerol | 0.074 | 44.5 | 3.29 | 15.21 | [56] |
| Corn | 0.112 | 36.7 | 3.72 | 19.13 | ||
| Potato | 0.055 | 31.6 | 6.56 | 5.67 | ||
| PV:PB (50:50, 60:40, 70:30, 80:20, 90:10, 100:0) | 0.061~0.070 | - | 27.5~52.6 | 108.1~241.8 | [57] | |
| NF | 0.064 | 13.06 | 3.49 | 19.21 | [58] | |
| ACT (4%, 8%) | 0.071, 0.072 | 14.47, 13.43 | 3.69, 2.86 | 31.4, 19.5 | ||
| HPS (10%, 30%) | 0.070, 0.067 | 16.49, 18.82 | 3.10, 2.54 | 57.17, 64.81 | ||
| Cassava starch | 5.5 | 45.5 | [59] | |||
| (5~15) wt% metakaolin+ glycerol | 5.7~8.1 | 23.1~33.2 | ||||
| Rice starch | sorbitol | 10.75 | 7.56 | [60] | ||
| (10~50) % NaOH+ sorbitol | 2.75~9.87 | 11.36~53.03 | ||||
| Octenyl succinate starch | glycerol | 0.087 | 29.54 | 9.60 | 32.41 | [61] |
| (0.025~0.100) % PSE+ glycerol | 0.090~0.091 | 29.22~29.62 | 7.56~8.62 | 23.99~30.98 | ||
| (0.025~0.100) % HSE+ glycerol | 0.090~0.091 | 29.74~30.03 | 7.31~10.58 | 29.58~31.65 |
| Starch | Additives | Product | Finding | References |
|---|---|---|---|---|
| Yam starch | eugenol | pork preservation | with 3% eugenol can extend the shelf-life of pork beyond 50% | [149] |
| Job’s tears starch | clove bud essential oil | pork belly | with 0.5% CBEO can reduce Lipid oxidation | [150] |
| Potato starch | carrot anthocyanins | row milk | used as an indicator to monitor freshness/spoilage of milk | [151] |
| Cassava starch | gelatin and casein | guavas | increased the guavas shelf-life by 2 days | [152] |
| Maize starch | grape juice | chicken breast fillets | delayed the lipid oxidation and microbiological growth of chicken breast fillets. | [153] |
| Brazilian pine seed starch | citric pectin and functionalized | grapes and bread | maintained the quality for 30 days of storage | [154] |
| Hydroxypropyl distarch phosphate | ε-polylysine and gelatin | fresh bread | delayed microbial spoilage | [95] |
| Corn starch | carboxymethyl cellulose | food simulant | excellent antimicrobial activity towards E. coli. | [155] |
| Potato starch | betacyanin | fish | visual change from pink to yellow color of the package label paralleled the increase in total volatile base nitrogen (TVB-N) | [156] |
| Corn starch | curcumin-loaded Pickering emulsion | fish | the color of films changed from yellow to red | [157] |
| Cassava starch | lycium ruthenicum anthocyanins-loaded nano-complexes | micropterus salmoides | when the fillet of perch deteriorates, the film shows significant color change | [158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhao, P.; Chen, J.; Yan, Y.; Wu, Z. Recent Advances and Applications in Starch for Intelligent Active Food Packaging: A Review. Foods 2022, 11, 2879. https://doi.org/10.3390/foods11182879
Liu D, Zhao P, Chen J, Yan Y, Wu Z. Recent Advances and Applications in Starch for Intelligent Active Food Packaging: A Review. Foods. 2022; 11(18):2879. https://doi.org/10.3390/foods11182879
Chicago/Turabian StyleLiu, Dandan, Pei Zhao, Jinyu Chen, Yali Yan, and Zijian Wu. 2022. "Recent Advances and Applications in Starch for Intelligent Active Food Packaging: A Review" Foods 11, no. 18: 2879. https://doi.org/10.3390/foods11182879
APA StyleLiu, D., Zhao, P., Chen, J., Yan, Y., & Wu, Z. (2022). Recent Advances and Applications in Starch for Intelligent Active Food Packaging: A Review. Foods, 11(18), 2879. https://doi.org/10.3390/foods11182879