Isotope Geochemistry for Seafood Traceability and Authentication: The Northern Adriatic Manila Clams Case Study
Abstract
:1. Introduction
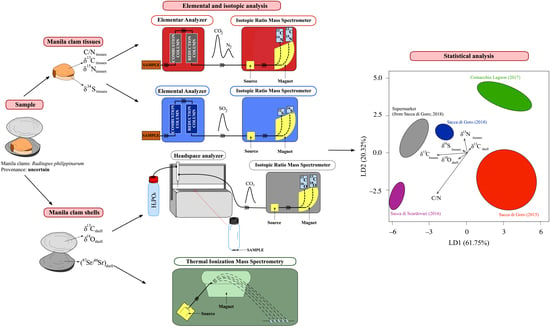
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Preparation of the Samples and Measurement Methods
2.3.1. C, N, and S Elemental and Isotopic Analyses of Manila Clam Tissues
2.3.2. C and O Isotopic Analyses of Manila Clam Shells
2.3.3. Sr Isotopic Analyses of Manila Clam Shells
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. C and N Isotopic Ratios of Manila Clam Tissues
4.2. S Isotopic Ratios of Manila Clam Tissues
4.3. C and O Isotopic Ratios of Manila Clam Shells
4.4. Sr Isotopic Ratios of Manila Clam Shells
4.5. Multivariate Analyses for the Traceability of Manila Clams at the Local Scale
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Fox, M.; Mitchell, M.; Dean, M.; Elliot, C.; Campbell, K. The seafood supply chain from a fraudulent perspective. Food Secur. 2018, 10, 939–963. [Google Scholar] [CrossRef] [Green Version]
- Oehlenschläger, J. Seafood: Nutritional benefits and risk aspects. Int. J. Vitam. Nutr. Res. 2012, 82, 168–176. [Google Scholar] [CrossRef]
- Camin, F.; Bontempo, L.; Heinrich, K.; Horacek, M.; Kelly, S.D.; Schlicht, C.; Thomas, F.; Monahan, F.J.; Hoogewerff, J.; Rossmann, A. Multi-element (H, C, N, S) stable isotope characteristics of lamb meat from different European regions. Anal. Bioanal. Chem. 2007, 389, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Schellenberg, A.; Chmielus, S.; Schlicht, C.; Camin, F.; Perini, M.; Bontempo, L.; Heinrich, K.; Kelly, S.D.; Rossmann, A.; Thomas, F.; et al. Multielement stable isotope ratios (H, C, N, S) of honey from different European regions. Food Chem. 2010, 121, 770–777. [Google Scholar] [CrossRef]
- Marchionni, S.; Braschi, E.; Tommasini, S.; Bollati, A.; Cifelli, F.; Mulinacci, N.; Mattei, M.; Conticelli, S. High-precision 87Sr/86Sr analyses in wines and their use as a geological fingerprint for tracing geographic provenance. J. Agric. Food Chem. 2013, 61, 6822–6831. [Google Scholar] [CrossRef]
- Braschi, E.; Marchionni, S.; Priori, S.; Casalini, M.; Tommasini, S.; Natarelli, L.; Buccianti, A.; Bucelli, P.; Costantini, E.A.C.; Conticelli, S. From vine to wine: Data on 87Sr/86Sr from rocks and soils as a geologic and pedologic characterization of vineyards. Sci. Total Environ. 2018, 18, 731–735. [Google Scholar] [CrossRef]
- Durante, C.; Baschieri, C.; Bertacchini, L.; Cocchi, M.; Sighinolfi, S.; Silvestri, M.; Marchetti, A. Geographical traceability based on 87Sr/86Sr indicator: A first approach for PDO Lambrusco wines from Modena. Food Chem. 2013, 141, 2779–2787. [Google Scholar] [CrossRef]
- Mimmo, T.; Camin, F.; Bontempo, L.; Capici, C.; Tagliavini, M.; Cesco, S.; Scampicchio, M. Traceability of different apple varieties by multivariate analysis of isotope ratio mass spectrometry data. Rapid Commun. Mass Spectrom. 2015, 29, 1984–1990. [Google Scholar] [CrossRef]
- Tescione, I.; Casalini, M.; Marchionni, S.; Braschi, E.; Mattei, M.; Conticelli, S. Conservation of 87Sr/86Sr during wine-making of white wines: A geochemical fingerprint of geographical provenance and quality production. Front. Environ. Sci. 2020, 8, 153. [Google Scholar] [CrossRef]
- Won, E.-J.; Kim, S.H.; Go, Y.-S.; Kumar, K.S.; Kim, M.-S.; Yoon, S.-H.; Bayon, G.; Kim, J.-H.; Shin, K.-H. A Multi-Elements Isotope Approach to Assess the Geographic Provenance of Manila Clams (Ruditapes philippinarum) via Recombining Appropriate Elements. Foods 2021, 10, 646. [Google Scholar] [CrossRef] [PubMed]
- Turolla, E.; Castaldelli, G.; Fano, E.A.; Tamburini, E. Life Cycle Assessment (LCA) Proves that Manila Clam Farming (Ruditapes Philippinarum) is a fully sustainable aquaculture practice and a carbon sink. Sustainability 2020, 12, 5252. [Google Scholar] [CrossRef]
- Bianchini, G.; Brombin, V.; Carlino, P.; Mistri, E.; Natali, C.; Salani, G.M. Traceability and authentication of manila clams from North-Western adriatic lagoons using C and N stable isotope analysis. Molecules 2021, 26, 1859. [Google Scholar] [CrossRef] [PubMed]
- Marchina, C.; Bianchini, G.; Natali, C.; Pennisi, M.; Colombani, N.; Tassinari, R.; Knoeller, K. The Po river water from the Alps to the Adriatic Sea (Italy): New insights from geochemical and isotopic (δ18O-δD) data. Environ. Sci. Pollut. Res. 2015, 22, 5184–5203. [Google Scholar] [CrossRef]
- Marchina, C.; Bianchini, G.; Knoeller, K.; Natali, C.; Pennisi, M.; Colombani, N. Natural and anthropogenic variations in the Po river waters (northern Italy): Insights from a multi-isotope approach. Isot. Environ. Health Stud. 2016, 52, 649–672. [Google Scholar] [CrossRef]
- Marchina, C.; Bianchini, G.; Natali, C.; Knoller, K. Geochemical and isotopic analyses on the Po delta water: Insights to understand a complex riverine ecosystem. Rend. Lincei Sci. Fis. Nat. 2016, 27, 83–88. [Google Scholar] [CrossRef]
- Yokoyama, H.; Ishihi, Y. Variation in δ13C and δ15N among different tissues of three estuarine bivalves: Implications for dietary reconstructions. Plankton Benthos Res. 2006, 1, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Wierzbowski, H. Effects of pre-treatments and organic matter on oxygen and carbon isotope analyses of skeletal and inorganic calcium carbonate. Int. J. Mass Spectrom. 2007, 268, 16–29. [Google Scholar] [CrossRef]
- Kusaka, S.; Nakano, T. Carbon and oxygen isotope ratios and their temperature dependence in carbonate and tooth enamel using GasBench II preparation device. Rapid Commun. Mass Spectrom. 2014, 28, 563–567. [Google Scholar] [CrossRef]
- Natali, C.; Bianchini, G. Thermally based isotopic speciation of carbon in complex matrices: A tool for environmental investigation. Environ. Sci. Pollut. Res. 2015, 22, 12162–12173. [Google Scholar] [CrossRef]
- Beccaluva, L.; Bianchini, G.; Natali, C.; Siena, F. The alkaline-carbonatite complex of Jacupiranga (Brazil): Magma genesis and mode of emplacement. Gondwana Res. 2017, 44, 157–177. [Google Scholar] [CrossRef]
- Dutta, K.; Schuur, E.A.G.; Neff, J.C.; Zimov, S.A. Potential carbon release from permafrost soils of Northeastern Siberia. Glob. Change Biol. 2006, 12, 2336–2351. [Google Scholar] [CrossRef]
- Coplen, T.B.; Qi, H. USGS42 and USGS43: Human-hair stable hydrogen and oxygen isotopic reference materials and analytical methods for forensic science and implications for published measurement results. Forensic Sci. Int. 2011, 214, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Halas, S.; Szaran, J. Improved thermal decomposition of sulfates to SO2 and mass spectrometric determinations of δ34S of IAEA-SO-5, IAEA-SO-6 and NBS-127 sulfate standards. Rapid Commun. Mass Spectrom. 2001, 15, 1618–1620. [Google Scholar] [CrossRef]
- Avanzinelli, R.; Boari, E.; Conticelli, S.; Francalanci, L.; Guarnieri, L.; Perini, G.; Petrone, C.M.; Tommasini, S.; Ulivi, M. High precision Sr, Nd, and Pb isotopic analyses using the new generation thermal ionisation mass spectrometer ThermoFinnigan Triton-Ti®. Period. Mineral. 2005, 74, 147–166. [Google Scholar]
- Weis, D.; Kieffer, B.; Maerschalk, C.; Barling, J.; De Jong, J.; Williams, G.A.; Hanano, D.; Pretorius, W.; Mattielli, N.; Scoates, J.S.; et al. High-precision isotopic characterization of USGS reference materials by TIMS and MC-ICP-MS. Geochem. Geophys. Geosyst. 2006, 7, Q08006. [Google Scholar] [CrossRef] [Green Version]
- Thirlwall, M.F. Long-term reproducibility of multicollector Sr and Nd isotope ratio analysis. Chem. Geol. 1991, 94, 85–104. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R--project.org/ (accessed on 22 June 2020).
- Briant, N.; Savoye, N.; Chovelon, T.; David, V.; Rodriguez, S.; Charlier, K.; Sonke, J.E.; Chiffoleau, J.F.; Brach-Papa, C.; Knoery, J. Carbon and nitrogen elemental and isotopic ratios of filter-feeding bivalves along the French coasts: An assessment of specific, geographic, seasonal and multi-decadal variations. Sci. Total Environ. 2018, 613, 196–207. [Google Scholar] [CrossRef]
- Dias, E.; Chainho, P.; Barrocas-Dias, C.; Adão, H. Food sources of the non-indigenous bivalve Ruditapes philippinarum (Adams and Reeve, 1850) and trophic niche overlap with native species. Aquat. Invasions 2019, 14, 638–655. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Zhang, J.; Liu, C.-Q. Strontium isotopic compositions of dissolved and suspended loads from the main channel of the Yangtze River. Chemosphere 2007, 69, 1081–1088. [Google Scholar] [CrossRef]
- Tulli, F.; Moreno-Rojas, J.M.; Messina, C.M.; Trocino, A.; Xiccato, G.; Muñoz-Redondo, J.M.; Santulli, A.; Tibaldi, E. The use of stable isotope ratio analysis to trace European sea bass (D. Labrax) originating from different farming systems. Animals 2020, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.C.; Mazumder, D.; Gadd, P.; Doubleday, Z.A. Tracking the provenance of octopus using isotopic and multi-elemental analysis. Food Chem. 2022, 371, 131133. [Google Scholar] [CrossRef] [PubMed]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, X.; Yang, F. Food sources of the Manila clam Ruditapes philippinarum in intertidal areas: Evidence from stable isotope analysis. Chin. J. Oceanol. Limnol. 2013, 31, 782–788. [Google Scholar] [CrossRef]
- Komorita, T.; Kajihara, R.; Tsutsumi, H.; Shibanuma, S.; Yamada, T.; Montani, S. Food sources for Ruditapes philippinarum in a coastal lagoon determined by mass balance and stable isotope approaches. PLoS ONE 2014, 9, e86732. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, L.; Nasi, F.; Florentino, F.; Auriemma, R.; Rampazzo, F.; Nordström, M.C.; Berto, D. Contribution of deltaic wetland food sources to coastal macrobenthic consumers (Po River Delta, north Adriatic Sea). Sci. Total Environ. 2018, 643, 1373–1386. [Google Scholar] [CrossRef]
- Berto, D.; Rampazzo, F.; Noventa, S.; Cacciatore, F.; Gabellini, M.; Bernardi Aubry, F.; Girolimetto, A.; Boscolo Brusà, R. Stable carbon and nitrogen isotope ratios as tools to evaluate the nature of particulate organic matter in the Venice lagoon. Estuar. Coast. Shelf Sci. 2013, 135, 66–76. [Google Scholar] [CrossRef]
- Yokoyama, H.; Tamaki, A.; Harada, K.; Shimoda, K.; Koyama, K.; Ishihi, Y. Variability of diet-tissue isotopic fractionation in estuarine macrobenthos. Mar. Ecol. Prog. Ser. 2005, 296, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Abbiati, M.; Mistri, M.; Bartoli, M.; Ceccherelli, V.U.; Colangelo, M.A.; Ferrari, C.R.; Giordani, G.; Munari, C.; Nizzoli, D.; Ponti, M.; et al. Trade-off between conservation and exploitation of the transitional water ecosystems of the northern Adriatic Sea. Chem. Ecol. 2010, 26, 105–119. [Google Scholar] [CrossRef]
- Mistri, M.; Fano, E.A.; Rossi, G.; Caselli, K.; Rossi, R. Variability in macrobenthos communities in the Valli di Comacchio, Northern Italy, a hypereutrophized lagoonal eco system. Estuar. Coast. Shelf Sci. 2000, 51, 599–611. [Google Scholar] [CrossRef]
- Martinelli, G.; Dadomo, A.; De Luca, D.A.; Mazzola, M.; Lasagna, M.; Pennisi, M.; Pilla, G.; Sacchi, E.; Saccon, P. Nitrate sources, accumulation and reduction in groundwater from Northern Italy: Insights provided by a nitrate and boron isotopic database. Appl. Geochem. 2018, 91, 23–35. [Google Scholar] [CrossRef]
- Watanabe, S.; Katayama, S.; Kodama, M.; Cho, N.; Nakata, K.; Fukuda, M. Small-scale variation in feeding environments for the Manila clam Ruditapes philippinarum in a tidal flat in Tokyo Bay. Fish. Res. 2009, 75, 937–945. [Google Scholar] [CrossRef]
- Suh, Y.J.; Shin, K.-H. Size-related and seasonal diet of the manila clam (Ruditapes philippinarum), as determined using dual stable isotopes. Estuar. Coast. Shelf Sci. 2013, 135, 94–105. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Hesslein, R.H.; Hallard, K.A.; Ramlal, P. Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Can. J. Fish. Aquat. Sci. 1993, 50, 2071. [Google Scholar] [CrossRef]
- Pinzone, M.; Acquarone, M.; Huyghebaert, L.; Sturaro, N.; Michel, L.N.; Siebert, U.; Das, K. Carbon, nitrogen and sulphur isotopic fractionation in captive juvenile hooded seal (Cystophora cristata): Application for diet analysis. Rapid Commun. Mass Spectrom. 2017, 31, 1720–1728. [Google Scholar] [CrossRef] [Green Version]
- Connolly, R.M.; Guest, M.A.; Melville, A.J.; Oakes, J.M. Sulfur stable isotopes separate producers in marine food-web analysis. Oecologia 2004, 138, 161–167. [Google Scholar] [CrossRef]
- Godbout, L.; Trudel, M.; Irvine, J.R.; Wood, C.C.; Grove, M.J.; Schmitt, A.K.; McKeegan, K.D. Sulfur isotopes in otoliths allow discrimination of anadromous and non-anadromous ecotypes of sockeye salmon (Oncorhynchus nerka). Environ. Biol. Fishes 2010, 89, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Nehlich, O.; Borić, D.; Stefanović, S.; Richards, M.P. Sulphur isotope evidence for freshwater fish consumption: A case study from the Danube Gorges, SE Europe. J. Archaeol. Sci. 2010, 37, 1131–1139. [Google Scholar] [CrossRef]
- Burke, A.; Present, T.M.; Paris, G.; Rae, E.C.M.; Sandilands, B.H.; Gaillardet, J.; Peucker-Ehrenbrink, B.; Fischer, W.W.; McClelland, J.M.; Spencer, R.G.M.; et al. Sulfur isotopes in rivers: Insights into global weathering budgets, pyrite oxidation, and the modern sulfur cycle. Earth Planet. Sci. Lett. 2018, 496, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; Mayers, B.; Bailey, S.W.; Hornbeck, J.W.; Alewell, C.; Driscoll, C.T.; Likens, G.E. Use of stable isotope ratios for evaluating sulfur sources and losses at the hubbard brook experimental forest. Water Air Soil Poll. 2001, 130, 75–86. [Google Scholar] [CrossRef]
- Giani, M.; Berto, D.; Zangrando, V.; Castelli, S.; Sist, P.; Urbani, R. Chemical characterization of different typologies of mucilaginous aggregates in the Northern Adriatic Sea. Sci. Total Environ. 2005, 353, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Borrell, A.; Gazo, M.; Aguilar, A.; Raga, J.A.; Degollada, E.; Gozalbes, P.; García-Vernet, R. Niche partitioning amongst northwestern Mediterranean cetaceans using stable isotopes. Prog. Oceanogr. 2021, 193, 102559. [Google Scholar] [CrossRef]
- Salani, G.M.; Brombin, V.; Natali, C.; Bianchini, G. Carbon, nitrogen, and sulphur isotope analysis of the Padanian Plain sediments: Backgrounds and provenance indication of the alluvial components. Appl. Geochem. 2021, 135, 105130. [Google Scholar] [CrossRef]
- Holmer, M.; Hasler-Sheetal, H. Sulfide intrusion in seagrasses assessed by stable sulfur isotopes-a synthesis of current results. Front. Mar. Sci. 2014, 1, 64. [Google Scholar] [CrossRef] [Green Version]
- Natali, C.; Bianchini, G. Geochemical proxies of sediment provenance in alluvial plains with interfering fluvial systems: A study case from NE Italy. Catena 2017, 157, 67–74. [Google Scholar] [CrossRef]
- Coletta, P.; Pentecost, A.; Spiro, B. Stable isotopes in charophyte incrustations: Relationships with climate and water chemistry. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 173, 9–19. [Google Scholar] [CrossRef]
- Kaandorp, R.J.G.; Vonhof, H.B.; Del Busto, C.; Wesselingh, F.P.; Ganssen, G.M.; Marmól, A.E.; Romero Pittman, L.; van Hinte, J.E. Seasonal stable isotope variations of the modern Amazonian freshwater bivalve Anodontites trapesialis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 194, 339–354. [Google Scholar] [CrossRef]
- Andrews, J.E.; Coletta, P.; Pentecost, A.; Riding, R.; Dennis, S.; Dennis, P.F.; Spiro, B. Equilibrium and disequilibrium stable isotope effects in modern charophyte calcites: Implications for palaeoenvironmental studies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 204, 101–114. [Google Scholar] [CrossRef]
- Shanahan, T.M.; Pigati, J.; Dettman, D.L.; Quade, J. Isotopic variability in the aragonite shells of freshwater gastropods living in springs with nearly constant temperature and isotopic composition. Geochim. Cosmochim. Acta 2005, 69, 3949–3966. [Google Scholar] [CrossRef]
- Poulain, C.; Lorrain, A.; Mas, R.; Gillikin, D.P.; Dehairs, F.; Robert, R.; Paulet, Y.-M. Experimental shift of diet and DIC stable carbon isotopes: Influence on shell 13C values in the Manila clam Ruditapes philippinarum. Chem. Geol. 2010, 272, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, P.F.; Lamothe, K.G. Seawater-buffered diagenesis, destruction of carbon isotope excursions, and the composition of DIC in Neoproterozoic oceans. Proc. Natl. Acad. Sci. USA 2019, 116, 18874–18879. [Google Scholar] [CrossRef] [Green Version]
- Castorina, F.; Vaiani, S.C. Riverine influence in Sr isotope ratio of mollusk shells and relationship with foraminiferal assemblages in a late Quaternary succession of the Po River Delta (northern Italy). Ital. J. Geosci. 2018, 137, 31–37. [Google Scholar] [CrossRef]
- Marchina, C.; Natali, C.; Fahnestock, F.; Pennisi, M.; Bryce, J.; Bianchini, G. Strontium isotopic composition of the Po river dissolved load: Insights into rock weathering in Northern Italy. Appl. Geochem. 2018, 97, 187–196. [Google Scholar] [CrossRef]
- Lugli, F.; Cipriani, A.; Bruno, L.; Ronchetti, F.; Cavazzuti, C.; Benazzi, S. A strontium isoscape of Italy for provenance studies. Chem. Geol. 2022, 587, 120624. [Google Scholar] [CrossRef]
- Allègre, C.J.; Louvat, P.; Gaillardet, J.; Meynadier, L.; Rad, S.; Capmas, F. The fundamental role of island arc weathering in the oceanic Sr isotope budget. Earth Planet. Sci. Lett. 2010, 292, 51–56. [Google Scholar] [CrossRef]
- Huang, K.-F.; You, C.-F.; Chung, C.-H.; Lin, I.-T. Nonhomogeneous seawater Sr isotopic composition in the coastal oceans: A novel tool for tracing water masses and submarine groundwater discharge. Geochem. Geophys. Geosyst. 2011, 12, Q05002. [Google Scholar] [CrossRef] [Green Version]
- Bernat, M.; Church, T.; Allègre, C.J. Barium and strontium concentrations in Pacific and Mediterranean sea water profiles by direct isotope dilution mass spectrometry. Earth Planet. Sci. Lett. 1972, 16, 75–80. [Google Scholar] [CrossRef]
- Mao, Y.; Lin, F.; Fang, J.; Fang, J.; Li, J.; Du, M. Bivalve Production in China. In Goods and Services of Marine Bivalves; Smaal, A., Ferreira, J., Grant, J., Petersen, J., Strand, Ø., Eds.; Springer: Cham, Switzerland, 2019; pp. 51–72. [Google Scholar]
- Wu, L.; Huh, Y.; Qin, J.; Du, G.; van Der Lee, S. Chemical weathering in the Upper Huang He (Yellow River) draining the eastern Qinghai-Tibet Plateau. Geochim. Cosmochim. Acta 2005, 69, 5279–5294. [Google Scholar] [CrossRef]
- Wang, J.; Chapman, D.; Xu, J.; Wang, Y.; Gu, B. Isotope niche dimension and trophic overlap between bigheaded carps and native filter-feeding fish in the lower Missouri River, USA. PLoS ONE 2018, 13, e0197584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Tang, Z. The first large-scale bioavailable Sr isotope map of China and its implication for provenance studies. Earth Sci. Rev. 2020, 210, 103353. [Google Scholar] [CrossRef]
- Kuznetsov, A.B.; Semikhatov, M.A.; Gorokhova, I.M. The Sr Isotope Composition of the World Ocean, Marginal and Inland Seas: Implications for the Sr Isotope Stratigraphy. Stratigr. Geol. Correl. 2012, 20, 501–515. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised pattern recognition in food analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef]
- Cuchet, A.; Anchisi, A.; Telouk, P.; Yao, Y.; Schiets, F.; Fourel, F.; Clément, Y.; Lantéri, P.; Carénini, E.; Jame, P.; et al. Multi-element (13C, 2H and 34S) bulk and compound-specific stable isotope analysis for authentication of Allium species essential oils. Food Control 2021, 126, 108086. [Google Scholar] [CrossRef]







| Lagoon | Sample | Tissues | Shell | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C (wt%) | N (wt%) | C/N | S (wt%) | δ13C (‰) | δ15N (‰) | δ34S (‰) | δ13C (‰) | δ18O (‰) | 87Sr/86Sr ± 2σ | ||
| Sacca di Goro (2015) | |||||||||||
| G10 | 34.10 | 7.33 | 4.65 | 0.87 | −24.0 | +7.3 | +17.5 | −5.0 | −3.6 | 0.709178 ± 0.000007 | |
| G1 | 35.80 | 7.96 | 4.50 | 1.20 | −22.9 | +8.1 | +13.6 | −5.1 | −3.5 | ||
| G2 | 38.30 | 7.92 | 4.84 | 0.99 | −23.6 | +8.9 | +16.7 | −5.2 | −4.1 | ||
| G3 | 33.40 | 7.54 | 4.43 | 0.74 | −23.8 | +10.0 | +13.5 | −5.0 | −4.0 | ||
| G4 | 36.80 | 8.18 | 4.50 | 1.05 | −23.8 | +9.6 | +16.6 | −5.8 | −3.9 | 0.709176 ± 0.000007 | |
| G5 | 39.40 | 8.21 | 4.80 | 0.91 | −24.7 | +10.4 | +17.2 | −5.6 | −3.4 | ||
| G6 | 40.30 | 8.77 | 4.60 | 1.15 | −24.1 | +11.0 | +17.3 | ||||
| G7 | 32.20 | 7.45 | 4.32 | 0.98 | −22.7 | +9.7 | +15.6 | −5.1 | −3.5 | ||
| G8 | 34.20 | 7.86 | 4.35 | 0.93 | −23.7 | +10.2 | +16.7 | −4.8 | −3.7 | 0.709163 ± 0.000007 | |
| G9 | 36.70 | 7.67 | 4.78 | 0.77 | −23.7 | +11.4 | +17.1 | −4.8 | −3.2 | ||
| Sacca di Goro (2018) | |||||||||||
| GA | 35.80 | 8.87 | 4.04 | 1.44 | −21.5 | +10.5 | +19.2 | −4.4 | −3.4 | 0.709183 ± 0.000007 | |
| GB | 34.67 | 8.90 | 3.89 | 1.45 | −21.4 | +10.1 | +19.4 | −3.7 | −3.4 | ||
| GC | 39.80 | 9.62 | 4.14 | 1.32 | −21.7 | +10.4 | +19.9 | −3.6 | −3.7 | ||
| GD | 36.03 | 9.07 | 3.97 | 1.31 | −21.3 | +10.1 | +19.7 | −3.5 | −3.9 | 0.709168 ± 0.000007 | |
| Supermarket (from Sacca di Goro; 2018) | |||||||||||
| TA | 37.06 | 9.89 | 3.75 | 1.58 | −20.4 | +9.4 | +19.5 | −3.3 | −2.5 | ||
| TB | 32.79 | 8.45 | 3.88 | 1.85 | −20.2 | +9.3 | +20.5 | −4.0 | −2.9 | 0.709179 ± 0.000007 | |
| TC | 35.20 | 9.70 | 3.80 | 1.66 | −20.8 | +11.1 | +20.2 | −3.9 | −2.6 | ||
| TD | 35.22 | 9.20 | 3.83 | 1.79 | −20.2 | +9.4 | +19.8 | −4.3 | −3.9 | ||
| Sacca di Scardovari (2016) | |||||||||||
| FF1 | 36.90 | 7.63 | 4.84 | −20.4 | +7.2 | −3.4 | −3.0 | ||||
| FF2 | 37.24 | 7.68 | 4.85 | −21.9 | +7.9 | −3.5 | −2.9 | 0.709168 ± 0.000008 | |||
| FF4 | 31.35 | 6.90 | 4.54 | 1.19 | −20.4 | +8.8 | +18.3 | −2.7 | −2.9 | 0.709176 ± 0.000006 | |
| FF5 | 30.94 | 6.75 | 4.58 | 1.32 | −20.4 | +8.2 | +19.0 | −2.5 | −1.5 | 0.709184 ± 0.000006 | |
| Laguna di Comacchio (2017) | |||||||||||
| LC1 | 38.52 | 8.87 | 4.34 | 1.36 | −23.6 | +14.7 | +17.0 | −5.1 | −2.8 | 0.709177 ± 0.000007 | |
| LC2 | 38.65 | 7.95 | 4.86 | 1.07 | −24.7 | +15.2 | +14.2 | −4.7 | −3.3 | 0.709173 ± 0.000007 | |
| LC3 | 36.83 | 8.72 | 4.22 | 1.45 | −23.0 | +14.2 | +17.8 | −2.4 | −3.4 | 0.709177 ± 0.000005 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brombin, V.; Natali, C.; Frijia, G.; Schmitt, K.; Casalini, M.; Bianchini, G. Isotope Geochemistry for Seafood Traceability and Authentication: The Northern Adriatic Manila Clams Case Study. Foods 2022, 11, 3054. https://doi.org/10.3390/foods11193054
Brombin V, Natali C, Frijia G, Schmitt K, Casalini M, Bianchini G. Isotope Geochemistry for Seafood Traceability and Authentication: The Northern Adriatic Manila Clams Case Study. Foods. 2022; 11(19):3054. https://doi.org/10.3390/foods11193054
Chicago/Turabian StyleBrombin, Valentina, Claudio Natali, Gianluca Frijia, Katharina Schmitt, Martina Casalini, and Gianluca Bianchini. 2022. "Isotope Geochemistry for Seafood Traceability and Authentication: The Northern Adriatic Manila Clams Case Study" Foods 11, no. 19: 3054. https://doi.org/10.3390/foods11193054
APA StyleBrombin, V., Natali, C., Frijia, G., Schmitt, K., Casalini, M., & Bianchini, G. (2022). Isotope Geochemistry for Seafood Traceability and Authentication: The Northern Adriatic Manila Clams Case Study. Foods, 11(19), 3054. https://doi.org/10.3390/foods11193054