Effect of Ethanol on Preparation of Konjac Emulgel-Based Fat Analogue by Freeze-Thaw Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Konjac Emulgels
2.3. Water and Fat Binding Properties
2.4. Syneresis Rate Measurement
2.5. Measurement of pH
2.6. Colour Measurement
2.7. Mechanical Properties
2.8. Low Field NMR Transverse Relaxation (T2) Measurements (LF-NMR)
2.9. Morphology Observation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Syneresis Rate, Heating Loss and pH
3.2. Colour Analysis
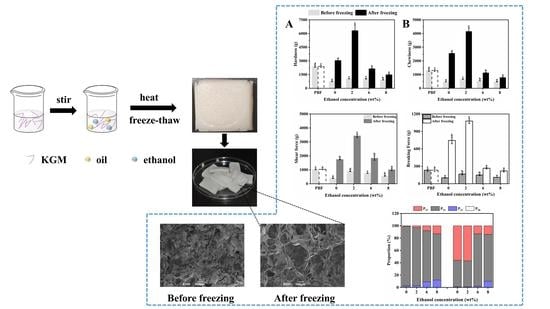
3.3. Mechanical Properties
3.4. LF-NMR Analysis
3.5. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.Y.; Li, C.M.; Regenstein, J.M.; Wang, L.F. Preparation and properties of potato amylose-based fat replacer using super-heated quenching. Carbohyd. Polym. 2019, 223, 115020. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Jin, W.; Zhou, B.; Li, B. Application of micronized konjac gel for fat analogue in mayonnaise. Food Hydrocoll. 2014, 35, 375–382. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zheng, H.; Li, Y.; Li, B.; Chen, Y.J. One step procedure for desalting salty egg white and preparing fat analogue and its application in mayonnaise. Food Hydrocoll. 2015, 45, 317–326. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, J.; Yu, D.; Kong, B.; Liu, Q. Recent Advances in the Application of Emulsion Gels as Fat Replacers in Meat Products. Food Sci. 2019, 40, 236–242. [Google Scholar] [CrossRef]
- Nishinari, K.; Williams, P.A.; Phillips, G.O. Review of the physicochemical characteristics and properties of konjacmannan. Food Hydrocoll. 1992, 6, 199–222. [Google Scholar] [CrossRef]
- Dave, V.; McCarthy, S.P. Review of Konjac glucomannan. J. Environ. Polym. Degrad. 1997, 5, 237–241. [Google Scholar]
- Yoshimura, M.; Nishinari, K. Dynamic viscoelastic study on the gelation of konjac glucomannan with different molecular weights. Food Hydrocoll. 1999, 13, 227–233. [Google Scholar] [CrossRef]
- Chua, M.; Baldwin, T.C.; Hocking, T.J.; Chan, K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex NEBr. J. Ethnopharmacol. 2010, 128, 268–278. [Google Scholar] [CrossRef]
- Lin, K.W.; Huang, H.Y. Konjac/gellan gum mixed gels improve the quality of reduced-fat frankfurters. Meat Sci. 2003, 65, 749–755. [Google Scholar] [CrossRef]
- Lin, K.W.; Huang, C.Y. Physicochemical and textural properties of ultrasound-degraded konjac flour and their influences on the quality of low-fat Chinese-style sausage. Meat Sci. 2008, 79, 615–622. [Google Scholar] [CrossRef]
- Jimenez-Colmenero, F.; Cofrades, S.; Lopez-Lopez, I.; Ruiz-Capillas, C.; Pintado, T.; Solas, M.T. Technological and sensory characteristics of reduced/low-fat, low-salt frankfurters as affected by the addition of konjac and seaweed. Meat Sci. 2010, 84, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Osburn, W.N.; Keeton, J.T. Evaluation of low-fat sausage containing desinewed lamb and konjac gel. Meat Sci. 2004, 68, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Sandoval, L.; Cofrades, S.; Perez, C.R.C.; Solas, M.T.; Jimenez-Colmenero, F. Healthier oils stabilized in konjac matrix as fat replacers in n-3 PUFA enriched frankfurters. Meat Sci. 2013, 93, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Colmenero, F.; Cofrades, S.; Herrero, A.M.; Fernandez-Martin, F.; Rodriguez-Salas, L.; Ruiz-Capillas, C. Konjac gel fat analogue for use in meat products: Comparison with pork fats. Food Hydrocoll. 2012, 26, 63–72. [Google Scholar] [CrossRef]
- Shang, L.; Wu, C.; Wang, S.; Wei, X.; Li, B.; Li, J. The influence of amylose and amylopectin on water retention capacity and texture properties of frozen-thawed konjac glucomannan gel. Food Hydrocoll. 2021, 113, 106521. [Google Scholar] [CrossRef]
- Zonoubi, R.; Goli, M. The effect of complete replacing sodium with potassium, calcium, and magnesium brine on sodium-free ultrafiltration Feta cheese at the end of the 60-day ripening period: Physicochemical, proteolysis-lipolysis indices, microbial, colorimetric, and sensory evaluation. Food Sci. Nutr. 2021, 9, 866–874. [Google Scholar] [CrossRef]
- Mirani, A.; Goli, M. Production of the eggplant-fiber incorporated cupcake and evaluating its chemical, textural and colorimetric properties over a ten-day storage time. J. Food Process. Pres. 2021, 45, e15311. [Google Scholar] [CrossRef]
- Nakao, Y.; Konno, A.; Taguchi, T.; Tawada, T.; Kasai, H.; Toda, J.; Terasaki, M. Curdlan—Properties and Application to Foods. J. Food Sci. 1991, 38, 736–742. [Google Scholar] [CrossRef]
- Zare, F.; Boye, J.I.; Orsat, V.; Champagne, C.; Simpson, B.K. Microbial, physical and sensory properties of yogurt supplemented with lentil flour. Food Res. Int. 2011, 44, 2482–2488. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J. Food texture and properties. J. Chin. Inst. Food Sci. Technol. 2021, 21, 377–384. [Google Scholar] [CrossRef]
- Genevro, G.M.; de Moraes, M.A.; Beppu, M.M. Freezing influence on physical properties of glucomannan hydrogels. Int. J. Biol. Macromol. 2019, 128, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.W.; Ke, F.; Xiao, M.; Wu, K.; Kuang, Y.; Corke, H.; Jiang, F.T. The control of ice crystal growth and effect on porous structure of konjac glucomannan-based aerogels. Int. J. Biol. Macromol. 2016, 92, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Arana, I. Physical Properties of Foods: Novel Measurement Techniques and Applications. In Physical Properties of Foods: Novel Measurement Techniques and Applications; Arana, I., Sun, D.W., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2012. [Google Scholar]
- Han, M.; Wang, P.; Xu, X.; Zhou, G. Low-field NMR study of heat-induced gelation of pork myofibrillar proteins and its relationship with microstructural characteristics. Food Res. Int. 2014, 62, 1175–1182. [Google Scholar] [CrossRef]






| Samples | Syneresis (%) | Heating Loss (%) | pH | Soybean Oil Content(wt%) |
|---|---|---|---|---|
| Pork backfat | —— | 1.01 ± 0.15 c | 6.30 ± 0.08 d | —— |
| 0 | 14.18 ± 0.77 a | 9.78 ± 0.71 a | 8.35 ± 0.04 c | 8.00 |
| 2 | 12.58 ± 0.47 b | 5.41 ± 0.58 b | 8.41 ± 0.05 c | 8.00 |
| 6 | 9.54 ± 0.72 c | 4.56 ± 0.22 b | 8.61 ± 0.04 b | 8.00 |
| 8 | 10.63 ± 0.46 c | 4.53 ± 0.62 b | 8.76 ± 0.06 a | 8.00 |
| Samples | L* | a* | b* | Chroma | ∆E | Whiteness Index | Browning Index |
|---|---|---|---|---|---|---|---|
| Pork backfat | 58.73 ± 0.76 a | −1.88 ± 0.10 c | 7.56 ± 1.01 a | 7.79 ± 1.22 a | — | 57.99 ± 1.04 a | 10.95 ± 2.30 a |
| KGFA before freezing | |||||||
| 0 | 44.38 ± 0.81 c | 1.13 ± 0.42 a | 1.21 ± 0.16 b | 1.68 ± 0.16 b | 15.98 ± 0.86 a | 44.36 ± 0.81 c | 4.48 ± 0.39 b |
| 2 | 47.97 ± 0.06 b | −0.16 ± 0.01 b | −2.67 ± 0.16 c | 2.67 ± 0.16 b | 14.95 ± 0.15 a | 47.91 ± 0.06 b | 5.43 ± 0.28 c |
| 6 | 49.09 ± 1.58 b | −0.36 ± 0.05 b | −2.28 ± 0.11 c | 2.30 ± 0.11 b | 13.87 ± 1.18 ab | 49.05 ± 1.58 b | 4.85 ± 0.30 c |
| 8 | 49.83 ± 0.95 b | −0.30 ± 0.03 b | −1.26 ± 0.04 c | 1.30 ± 0.02 b | 12.64 ± 0.69 b | 49.81 ± 0.95 b | 2.82 ± 0.07 c |
| Pork backfat | 58.73 ± 0.76 a | −1.88 ± 0.10 c | 7.56 ± 1.01 a | 7.79 ± 1.22 a | — | 57.99 ± 1.04 a | 10.95 ± 2.30 a |
| KGFA after freezing | |||||||
| 0 | 32.72 ± 0.11 d | 0.80 ± 0.05 a | −0.12 ± 0.06 b | 0.80 ± 0.06 c | 27.25 ± 0.13 a | 32.72 ± 0.11 d | 1.37 ± 0.06 b |
| 2 | 43.10 ± 0.93 c | −0.36 ± 0.07 b | −3.81 ± 0.15 c | 3.82 ± 0.16 b | 18.52 ± 2.06 b | 42.97 ± 0.93 c | 8.69 ± 0.59 a |
| 6 | 54.23 ± 0.98 b | −0.40 ± 0.13 b | −1.11 ± 0.16 b | 1.18 ± 0.18 c | 9.91 ± 0.34 c | 54.22 ± 0.97 b | 2.46 ± 0.38 b |
| 8 | 53.79 ± 1.23 b | −0.17 ± 0.08 b | −0.86 ± 0.24 b | 0.88 ± 0.25 c | 9.94 ± 0.80 c | 53.79 ± 1.24 b | 1.75 ± 0.57 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Zongo, A.W.-S.; Geng, F.; Li, J.; Li, B. Effect of Ethanol on Preparation of Konjac Emulgel-Based Fat Analogue by Freeze-Thaw Treatment. Foods 2022, 11, 3173. https://doi.org/10.3390/foods11203173
Jiang J, Zongo AW-S, Geng F, Li J, Li B. Effect of Ethanol on Preparation of Konjac Emulgel-Based Fat Analogue by Freeze-Thaw Treatment. Foods. 2022; 11(20):3173. https://doi.org/10.3390/foods11203173
Chicago/Turabian StyleJiang, Jie, Abel Wend-Soo Zongo, Fang Geng, Jing Li, and Bin Li. 2022. "Effect of Ethanol on Preparation of Konjac Emulgel-Based Fat Analogue by Freeze-Thaw Treatment" Foods 11, no. 20: 3173. https://doi.org/10.3390/foods11203173
APA StyleJiang, J., Zongo, A. W. -S., Geng, F., Li, J., & Li, B. (2022). Effect of Ethanol on Preparation of Konjac Emulgel-Based Fat Analogue by Freeze-Thaw Treatment. Foods, 11(20), 3173. https://doi.org/10.3390/foods11203173