A Review of the Current State and Future Prospects in Resource Recovery of Chinese Cereal Vinegar Residue
Abstract
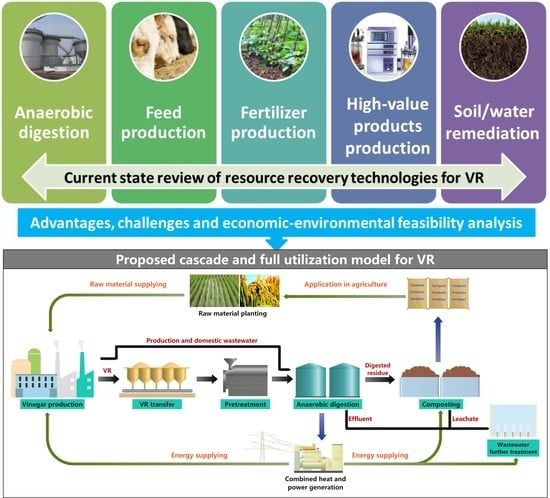
:1. Introduction
2. Resource Recovery Technologies of VR
2.1. Anaerobic Digestion
2.1.1. Advances in Anaerobic Digestion Research
2.1.2. Challenges in Anaerobic Digestion of VR
- (1)
- The biodegradability is low in the absence of pretreatment, which can lead to a gradual accumulation of lignocellulose in the anaerobic digestion reactor. The accumulation of lignocellulose further causes flushing of activated sludge from the reactor and depresses the performance of anaerobic digestion.
- (2)
- VR pretreatment methods also have inherent problems [67]. SE, hydrothermal and ultrasonic pretreatments require high energy consumption. Acid and alkali pretreatment can lead to secondary contamination and equipment corrosion. In addition, inhibitors produced during pretreatment, such as furan derivatives, can inhibit microorganisms in anaerobic digestion systems.
- (3)
- The residues remaining after VR anaerobic digestion need further treatment. Therefore, the optimized conditions and pre- and posttreatment of VR anaerobic digestion should be further investigated.
2.2. Feed Production
2.2.1. Advances in Feed Production
2.2.2. Challenges in Feed Production
- (1)
- Wet VR is prone to deterioration, which can result in the production of toxic mycotoxins, especially when transported over long distances without proper storage management. Livestock fed with spoiled VR can exhibit respiratory distress, diarrhea, and other toxicities. Therefore, wet VR should be dried before being transported to feed producers, but this process requires significant energy consumption.
- (2)
- The low energy concentration and density of VR makes livestock susceptible to satiety and further reduces feed intake. When livestock (particularly monogastric animals) were fed a high percentage of VR, the low digestibility caused by the high fiber content would speed up the passage of chyme through the intestine, thus reducing nutrient absorption [21,91].
- (3)
- Higher levels of alcohol left in VR can also pose a risk of poisoning in livestock. Higher levels of alcohol have been reported to cause metabolic disorders, liver disease and brain damage [92].
2.3. Fertilizer Production
2.3.1. Advances in Fertilizer Production
2.3.2. Challenges in Fertilizer Production
- (1)
- Gases (such as CO2, NH3 and N2O) are emitted during the composting process. These gases not only cause odor problems but also cause greenhouse issues [106]. In addition, NH3 emissions result in an excess of 70% of total nitrogen losses [107]. Therefore, process optimization should be performed to control gas emissions.
- (2)
- VR consists of bran, rice husk and other filling materials which contain highly crystalline lignocellulose that is recalcitrant to composting. High lignocellulose content in plant wastes has been reported to elongate the composting time in the composting pile. Therefore, to obtain good performance of composting for VR, a long composting period and a large land demand are needed [108].
2.4. High-Value Product Production
2.4.1. Advances in High-Value Product Production
2.4.2. Challenges in High-Value Product Production
- (1)
- VR pretreatment usually consumes large amounts of energy, chemicals and hydrolytic enzymes, thus greatly increasing the processing cost of the raw materials.
- (2)
- VR pretreatment can produce inhibitors to ferment microorganisms, so these inhibitors should be removed from the hydrolysate prior to fermentation, or highly inhibitor-tolerant microorganisms should be generated.
- (3)
- Acid and alkali pretreatment results in a large amount of wastewater that should be treated before being discharged.
- (4)
- The final concentration of the product at the end of fermentation is relatively lower than that of the conventional raw material, so the cost of product purification is high.
2.5. Soil/Water Remediation
2.5.1. Advances in Soil/Water Remediation
2.5.2. Challenges in Soil/Water Amendment Production
- (1)
- Although VR-supported nZVI has been effectively used for soil and water remediation, exposure to nZVI has harmful effects on humans and the environment [115].
- (2)
- Biochar made from VR has great advantages in soil remediation, but the long-term effects of biochar on soil remain unclear. Therefore, to reduce the possible risks associated with biochar, more attention should be given to the long-term effects and risk assessment of biochar on soil. For example, heavy metals immobilized on biochar may be rereleased due to chemical, physical and biological degradation caused by weathering aging [116].
3. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAF | Acetic acid fermentation |
| AB | Acetylene black |
| ARTP | Atmospheric and room-temperature plasma |
| BMP | Biochemical methane potential |
| BSG | Brewer’s spent grain |
| CFU | Colony forming units |
| C/N | Carbon-to-nitrogen ratio |
| CO2 | Carbon dioxide |
| Cr | Chromium |
| CSTR | Continuously stirred tank reactor |
| CS | Corn straw |
| DGS | Distiller’s grains |
| DIET | Direct interspecies electron transfer |
| EE | Ether extract |
| GI | Germination index |
| HC | Hydrochar |
| IU | International unit |
| MFF | Microbial fermented feed |
| ML | Machine learning |
| NA | Not Available |
| NaOH | Sodium hydroxide |
| NH3 | Ammonia |
| N2O | Nitrous oxide |
| nZVI | Nanoscale zero-valent iron |
| OLR | Organic loading rate |
| PB | Plackett–Burman |
| RSM | Response surface methodology |
| SE | Steam explosion |
| SSF | Solid-state fermentation |
| VFA | Volatile fatty acid |
| VR | Vinegar residue |
| VRB | VR biochar |
| VS | Volatile solid |
| ZnCl2 | Zinc chloride |
References
- Giudici, P.; Corradini, G.; Bonciani, T.; Jiajia, W.; Chen, F.; Lemmetti, F. The flavor and taste of cereal Chinese vinegars. Acetic Acid Bact. 2017, 6, 6370. [Google Scholar] [CrossRef]
- Chen, F.S.; Li, L.; Qu, J.; Chen, C.X. Cereal vinegars made by solid-state fermentation in China. In Vinegars of the World; Solieri, L., Giudici, P., Eds.; Springer: Milano, Italy, 2009; pp. 243–259. [Google Scholar]
- Xia, T.; Zhang, B.; Duan, W.H.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Li, L.; Feng, L.; Zhang, R.; He, Y.; Wang, W.; Chen, C.; Liu, G. Anaerobic digestion performance of vinegar residue in continuously stirred tank reactor. Bioresour. Technol. 2015, 186, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, G.N.; Zheng, T.; Zhou, Z.Z.; Wu, Q.G.; Yuan, H.R. Effect of the organic loading rates increase on process stability and microbial community composition during the anaerobic digestion of fresh vinegar residue. Waste Biomass Valori. 2021, 12, 5505–5516. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, J.; Zhang, J.; He, Y.; Zhang, R.; Chen, C.; Liu, G. Enhanced methane production of vinegar residue by response surface methodology (RSM). AMB Express 2017, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zhang, J.; Zhang, J.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Influence of steam explosion pretreatment on the anaerobic digestion of vinegar residue. Waste Manag. Res. 2016, 34, 630–637. [Google Scholar] [CrossRef]
- Feng, L.; Li, Y.Q.; Chen, C.; Liu, X.Y.; Xiao, X.; Ma, X.X.; Zhang, R.H.; He, Y.F.; Liu, G.Q. Biochemical methane potential (BMP) of vinegar residue and the influence of feed to inoculum ratios on biogas production. Bioresources 2013, 8, 2487–2498. [Google Scholar] [CrossRef]
- Guo, W.Y.; Li, Y.Q.; Zhao, K.; Xu, Q.; Jiang, H.; Zhou, H.J. Performance and microbial community analysis of anaerobic digestion of vinegar residue with adding of acetylene black or hydrochar. Waste Biomass Valori. 2019, 11, 3315–3325. [Google Scholar] [CrossRef]
- Kong, X.; Defemur, Z.; Li, M.; Zhang, Q.; Li, H.; Yue, X. Effects of combined ultrasonic and grinding pre-treatments on anaerobic digestion of vinegar residue: Organic solubilization, hydrolysis, and CH4 production. Environ. Technol. 2021, 43, 2207–2217. [Google Scholar] [CrossRef]
- Ran, G.; Li, D.; Zheng, T.; Liu, X.; Chen, L.; Cao, Q.; Yan, Z. Hydrothermal pretreatment on the anaerobic digestion of washed vinegar residue. Bioresour. Technol. 2018, 248, 265–271. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, C.; Liu, G.Q.; Chen, C. Enhancing the performance on anaerobic digestion of vinegar residue by sodium hydroxide pretreatment. Waste Biomass Valori. 2016, 8, 1119–1126. [Google Scholar] [CrossRef]
- Song, C.; Feng, J.Y.; Wang, L.G.; Cai, F.F.; Chen, C.; Liu, G.Q. Comparison of methane production performance of vinegar residue under liquid- and solid-state conditions. Environ. Prog. Sustain. 2020, 40, e13533. [Google Scholar] [CrossRef]
- Wang, L.G.; Feng, J.Y.; Cai, F.F.; Wang, J.; Chen, C.; Liu, G.Q. Anaerobic digestion and lignocellulosic compositions alteration of industrial vinegar residue after different pretreatments. Environ. Technol. Innov. 2021, 24, 101941. [Google Scholar] [CrossRef]
- Wang, Z.B.; Shao, S.P.; Zhang, C.S.; Lu, D.L.; Ma, H.L.; Ren, X.F. Pretreatment of vinegar residue and anaerobic sludge for enhanced hydrogen and methane production in the two-stage anaerobic system. Int. J. Hydrog. Energy 2015, 40, 4494–4501. [Google Scholar] [CrossRef]
- Wen, H.; Wachemo, A.C.; Zhang, L.; Zuo, X.; Yuan, H.; Li, X. Novel strategy for efficient anaerobic co-digestion based on the pretreatment of corn stover with fresh vinegar residue. Bioresour. Technol. 2019, 288, 121412. [Google Scholar] [CrossRef]
- Zhou, A.J.; Liu, Z.H.; Varrone, C.; Luan, Y.B.; Liu, W.Z.; Wang, A.J.; Yue, X.P. Efficient biorefinery of waste activated sludge and vinegar residue into volatile fatty acids: Effect of feedstock conditioning on performance and microbiology. Environ. Sci. Water Res. Technol. 2018, 4, 1819–1828. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Xu, Z.Y.; Zhao, M.X.; Shi, W.S.; Huang, Z.X.; He, D.; Ruan, W.Q. Construction and evaluation of efficient solid-state anaerobic digestion system via vinegar residue. Int. Biodeterior. Biodegrad. 2018, 133, 142–150. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Yang, J.; Zhang, W.; Wang, Q.; Zhang, J.; Xin, J.; Chen, S. Study on the nutritional value and ruminal degradation characteristics of fermented waste vinegar residue by N. sitophila. Trop. Anim. Health Prod. 2019, 51, 1449–1454. [Google Scholar] [CrossRef]
- Song, Z.T.; Dong, X.F.; Tong, J.M.; Wang, Z.H. In sacco evaluation of ruminal degradability of waste vinegar residue as a feedstuff for ruminants. Anim. Prod. Sci. 2013, 53, 292–298. [Google Scholar] [CrossRef]
- Song, Z.T.; Dong, X.F.; Tong, J.M.; Wang, Z.H. Effects of waste vinegar residue on nutrient digestibility and nitrogen balance in laying hens. Livest. Sci. 2012, 150, 67–73. [Google Scholar] [CrossRef]
- Liu, K.Y.; Jiang, B.; Wang, Q.; Yang, L.R.; Lu, B.; Li, X.P.; Yuan, H.W. Study on the conditions of pretreating vinegar residue with sodium hydroxide for simultaneous saccharification and fermentation to produce alcohol and xylose. Food Sci. Technol. Res. 2020, 26, 381–388. [Google Scholar] [CrossRef]
- Liu, K.Y.; Zhou, S.L.; Wang, Q.; Jiang, B.; Yang, L.R.; Lu, B.; Yuan, H.W. Enzymatic degradation of polysaccharides in Chinese vinegar residue to produce alcohol and xylose. Czech J. Food Sci. 2021, 39, 160–168. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, X.; Tong, J.; Wu, Y.; Zhang, Q. Vinegar production residue as substrates for phytase production by Aspergillus ficuum. Waste Manag. Res. 2010, 28, 165–168. [Google Scholar] [CrossRef]
- Wang, Z.H.; Dong, X.F.; Zhang, G.Q.; Tong, J.M.; Zhang, Q.; Xu, S.Z. Waste vinegar residue as substrate for phytase production. Waste Manag. Res. 2011, 29, 1262–1270. [Google Scholar] [CrossRef]
- Li, X.L. Research on Vinegar Residue Hydrolyzate Fermentation for Feed Microorganism Product. Master’s Thesis, Hubei University, Wuhan, China, 2015. [Google Scholar]
- Zhao, Q.S. Study on the Changes of Physiochemical Characteristics of Vinegar Residue Substrate under Long-Term Using and N, P, K Nutrient Management. Ph.D. Thesis, Jiangsu University, Zhenjiang, China, 2012. [Google Scholar]
- Pang, K.Y. Utilization of Vinegar Residue as Fertilizer in Calcareous Cinnamon Soils. Master’s Thesis, Shanxi Agricultural University, Shanxi, China, 2016. [Google Scholar]
- Du, N.; Shi, L.; Du, L.; Yuan, Y.; Li, B.; Sang, T.; Sun, J.; Shu, S.; Guo, S. Effect of vinegar residue compost amendments on cucumber growth and Fusarium wilt. Environ. Sci. Pollut. Res. Int. 2015, 22, 19133–19141. [Google Scholar] [CrossRef]
- Shi, L.; Du, N.; Yuan, Y.; Shu, S.; Sun, J.; Guo, S. Vinegar residue compost as a growth substrate enhances cucumber resistance against the Fusarium wilt pathogen Fusarium oxysporum by regulating physiological and biochemical responses. Environ. Sci. Pollut. Res. Int. 2016, 23, 18277–18287. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shah Jahan, M.; Wang, Y.; Sun, J.; Shu, S.; Guo, S. Compost amendments based on vinegar residue promote tomato growth and suppress bacterial wilt caused by Ralstonia Solanacearum. Pathogens 2020, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Xia, M.; Peng, M.; Xue, D.; Cheng, Y.; Li, C.; Wang, D.; Lu, K.; Zheng, Y.; Xia, T.; Song, J.; et al. Development of optimal steam explosion pretreatment and highly effective cell factory for bioconversion of grain vinegar residue to butanol. Biotechnol. Biofuels 2020, 13, 111. [Google Scholar] [CrossRef]
- Qiao, Y.B.; Qin, W.H.; Li, Q.S. Sulfates of sorghum vinegar residue waste as potential catalysts. Green Process. Synth. 2018, 7, 334–343. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Zhou, Z.Z.; Yuan, H.R.; Zheng, T. Effect of anaerobic pretreatment on vinegar residue for enhancement of syngas and phenols derived from pyrolysis. Asia-Pac. J. Chem. Eng. 2019, 14, e2342. [Google Scholar] [CrossRef]
- Pei, G.; Li, Y.; Zhu, Y.; Shi, W.; Li, H. Immobilization of lead by application of soil amendment produced from vinegar residue, stainless steel slag, and weathered coal. Environ. Sci. Pollut. Res. Int. 2017, 24, 22301–22311. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Zhu, Y.; Wen, J.; Pei, Y.; Li, H. Vinegar residue supported nanoscale zero-valent iron: Remediation of hexavalent chromium in soil. Environ. Pollut. 2020, 256, 113407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, J.Y.; Xiao, Z.X.; Tang, S.S.; Chen, Y.L.; Sun, G.Z.; Wang, W.D.; Yu, Y.D. Using vinegar residue-based carrier materials to improve the biodegradation of phenanthrene in aqueous solution. J. Nanosci. Nanotechnol. 2021, 21, 3134–3147. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pei, G.; Qiao, X.; Zhu, Y.; Li, H. Remediation of cadmium contaminated water and soil using vinegar residue biochar. Environ. Sci. Pollut. Res. Int. 2018, 25, 15754–15764. [Google Scholar] [CrossRef]
- Ding, K.; Zhou, X.; Hadiatullah, H.; Lu, Y.; Zhao, G.; Jia, S.; Zhang, R.; Yao, Y. Removal performance and mechanisms of toxic hexavalent chromium (Cr(VI)) with ZnCl2 enhanced acidic vinegar residue biochar. J. Hazard. Mater. 2021, 420, 126551. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. Performance evaluation of anaerobic digestion technology for energy recovery from organic fraction of municipal solid waste: A review. Energy 2020, 197, 117253. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Hong, Y.; La, D.D.; Nguyen, X.C.; Ngo, H.H.; et al. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Yu, M.; Wu, C.F.; Wang, Q.H.; Gao, M.; Huang, Q.Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Srisowmeya, G.; Chakravarthy, M.; Devi, G.N. Critical considerations in two-stage anaerobic digestion of food waste—A review. Renew. Sust. Energ. Rev. 2020, 119, 109587. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.Y.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.M.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, Y.Q.; Zheng, L.K.; Wang, Z.W.; Dai, X.H. Perspective on enhancing the anaerobic digestion of waste activated sludge. J. Hazard. Mater. 2020, 389, 121847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Thakur, N.; Salama, E.-S.; Zhang, P.; Zhang, L.H.; Xing, X.H.; Yue, J.W.; Song, Z.Z.; Nan, L.; Su, Y.J.; et al. Potential applications of protein-rich waste: Progress in energy management and material recovery. Resour. Conserv. Recy. 2022, 182, 106315. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Liu, S.P.; Huang, C.H.; Ge, X.Y.; Xi, B.D.; Mao, J. Chinese Baijiu distiller’s grains resourcing: Current progress and future prospects. Resour. Conserv. Recycl. 2022, 176, 105900. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef]
- Raposo, F.; Fernandez-Cegri, V.; De la Rubia, M.A.; Borja, R.; Beline, F.; Cavinato, C.; Demirer, G.; Fernandez, B.; Fernandez-Polanco, M.; Frigon, J.C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Eryildiz, B.; Lukitawesa; Taherzadeh, M.J. Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour. Technol. 2020, 302, 122800. [Google Scholar] [CrossRef]
- Jiang, Y.; Heaven, S.; Banks, C.J. Strategies for stable anaerobic digestion of vegetable waste. Renew. Energ. 2012, 44, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Luste, S.; Luostarinen, S. Anaerobic co-digestion of meat-processing by-products and sewage sludge—Effect of hygienization and organic loading rate. Bioresour. Technol. 2010, 101, 2657–2664. [Google Scholar] [CrossRef]
- Nagao, N.; Tajima, N.; Kawai, M.; Niwa, C.; Kurosawa, N.; Matsuyama, T.; Yusoff, F.M.; Toda, T. Maximum organic loading rate for the single-stage wet anaerobic digestion of food waste. Bioresour. Technol. 2012, 118, 210–218. [Google Scholar] [CrossRef]
- Storck, T.; Virdis, B.; Batstone, D.J. Modelling extracellular limitations for mediated versus direct interspecies electron transfer. ISME J. 2016, 10, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Kumar Khanal, S. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.G.; Imai, T.; Ukita, M.; Sekine, M. Start-up performances of dry anaerobic mesophilic and thermophilic digestions of organic solid wastes. J. Environ. Sci. 2007, 19, 416–420. [Google Scholar] [CrossRef]
- Noike, T.; Endo, G.; Chang, J.E.; Yaguchi, J.I.; Matsumoto, J.I. Characteristics of carbohydrate degradation and the rate-limiting step in anaerobic digestion. Biotechnol. Bioeng. 1985, 27, 1482–1489. [Google Scholar] [CrossRef]
- He, Y.F.; Pang, Y.Z.; Liu, Y.P.; Li, X.J.; Wang, K.S. Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels 2008, 22, 2775–2781. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.N.; Sanchez, A.; Rocha, G.J.M.; Romani, A.; Castro, E.; Torres, A.; Rodriguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef] [Green Version]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [Green Version]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- Sui, W.J.; Chen, H.Z. Water transfer in steam explosion process of corn stalk. Ind. Crop. Prod. 2015, 76, 977–986. [Google Scholar] [CrossRef]
- He, L.L.; Huang, H.; Zhang, Z.Y.; Lei, Z.F. A review of hydrothermal pretreatment of lignocellulosic biomass for enhanced biogas production. Curr. Org. Chem. 2015, 19, 437–446. [Google Scholar] [CrossRef]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.I. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef] [PubMed]
- Alipour, D.; Rouzbehan, Y. Effects of ensiling grape pomace and addition of polyethylene glycol on in vitro gas production and microbial biomass yield. Anim. Feed Sci. Technol. 2007, 137, 138–149. [Google Scholar] [CrossRef]
- Devendra, C.; Leng, R.A. Feed resources for animals in Asia: Issues, strategies for use, intensification and integration for increased productivity. Asian-Australas. J. Anim. Sci. 2011, 24, 303–321. [Google Scholar] [CrossRef]
- Negesse, T.; Makkar, H.P.S.; Becker, K. Nutritive value of some non-conventional feed resources of Ethiopia determined by chemical analyses and an in vitro gas method. Anim. Feed Sci. Technol. 2009, 154, 204–217. [Google Scholar] [CrossRef]
- Chuang, W.Y.; Lin, L.J.; Shih, H.D.; Shy, Y.M.; Chang, S.C.; Lee, T.T. The potential utilization of high-fiber agricultural by-products as monogastric animal feed and feed additives: A review. Animals 2021, 11, 2098. [Google Scholar] [CrossRef]
- Shee, C.N.; Lemenager, R.P.; Schoonmaker, J.P. Feeding dried distillers grains with solubles to lactating beef cows: Impact of excess protein and fat on cow performance, milk production and pre-weaning progeny growth. Animal 2016, 10, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, S.; Zebeli, Q.; Mazzolari, A.; Bertoni, G.; Dunn, S.M.; Yang, W.Z.; Ametaj, B.N. Feeding barley grain steeped in lactic acid modulates rumen fermentation patterns and increases milk fat content in dairy cows. J. Dairy Sci. 2009, 92, 6023–6032. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, S.; Zebeli, Q.; Mazzolari, A.; Dunn, S.M.; Ametaj, B.N. Feeding rolled barley grain steeped in lactic acid modulated energy status and innate immunity in dairy cows. J. Dairy Sci. 2010, 93, 5147–5156. [Google Scholar] [CrossRef]
- Montano, M.F.; Chai, W.; Zinn-Ware, T.E.; Zinn, R.A. Influence of malic acid supplementation on ruminal pH, lactic acid utilization, and digestive function in steers fed high-concentrate finishing diets. J. Anim. Sci. 1999, 77, 780–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.A.; Streeter, M.N.; Nisbet, D.J.; Hill, G.M.; Williams, S.E. Effects of DL-malate on ruminal metabolism and performance of cattle fed a high-concentrate diet. J. Anim. Sci. 1999, 77, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Rotz, C.A. Management to reduce nitrogen losses in animal production. J. Anim. Sci. 2004, 82 (Suppl. 13), E119–E137. [Google Scholar] [PubMed]
- Huang, L.L.; Ren, P.P.; Ouyang, Z.C.; Wei, T.; Kong, X.F.; Li, T.J.; Yin, Y.L.; He, S.P.; Yang, C.B.; He, Q.H. Effect of fermented feed on growth performance, holistic metabolism and fecal microbiota in weanling piglets. Anim. Feed Sci. Technol. 2020, 266, 114505. [Google Scholar] [CrossRef]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Deng, Y.P.; Xin, J.Y.; Liu, X.L.; Ai, R.B.; Wang, X.J. Optimization of culture conditions and medium composition for the synthesis of xylanase by resting Neurospora sitophila cells: Analysis of the effects of sugars on xylanase production using the resting cell. J. Biobased Mater. Bioenergy 2017, 11, 553–561. [Google Scholar] [CrossRef]
- Li, Y.J.; Peng, X.W.; Chen, H.Z. Comparative characterization of proteins secreted by Neurospora sitophila in solid-state and submerged fermentation. J. Biosci. Bioeng. 2013, 116, 493–498. [Google Scholar] [CrossRef]
- Djali, M.; Kayaputri, I.L.; Kurniati, D.; Sukarminah, E.; Mudjenan, I.M.H.; Utama, G.L. Degradation of lignocelluloses cocoa shell (Theobroma cacao) by various types of mould treatments. J. Food Qual. 2021, 2021, 6127029. [Google Scholar] [CrossRef]
- Moo-Young, M.; Chisti, Y.; Vlach, D. Fermentative conversion of cellulosic substrates to microbial protein by Neurospora sitophila. Biotechnol. Lett. 1992, 14, 863–868. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Duvnjak, Z. Effect of glucose concentration on the biomass and phytase productions and the reduction of the phytic acid content in canola meal by Aspergillus carbonarius during a solid-state fermentation process. Biotechnol. Prog. 1994, 10, 353–359. [Google Scholar] [CrossRef]
- Volfová, O.; Dvoráková, J.; Hanzlíková, A.; Jandera, A. Phytase from Aspergillus niger. Folia Microbiol. 1994, 39, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.M. Production of extracellular phytase from Aspergillus ficuum on starch media. Biotechnol. Lett. 1987, 9, 305–310. [Google Scholar] [CrossRef]
- Papagianni, M.; Nokes, S.E.; Filer, K. Production of phytase by Aspergillus niger in submerged and solid-state fermentation. Process Biochem. 2002, 35, 397–402. [Google Scholar] [CrossRef]
- Haefner, S.; Knietsch, A.; Scholten, E.; Braun, J.; Lohscheidt, M.; Zelder, O. Biotechnological production and applications of phytases. Appl. Microbiol. Biotechnol. 2005, 68, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.K.; Kim, W.S.; Paik, H.D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]
- Jørgensen, H.; Zhao, X.Q.; Eggum, B.O. The influence of dietary fibre and environmental temperature on the development of the gastrointestinal tract, digestibility, degree of fermentation in the hind-gut and energy metabolism in pigs. Br. J. Nutr. 1996, 75, 365–378. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Haupt, S.; Schulz, K. Defining maximum levels of higher alcohols in alcoholic beverages and surrogate alcohol products. Regul. Toxicol. Pharmacol. 2008, 50, 313–321. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.C.; Zhang, J.B.; Fan, J.; Yang, X.Y.; Yi, Y.L.; Han, X.R.; Wang, D.Z.; Zhu, P.; Peng, X.H. Does animal manure application improve soil aggregation? Insights from nine long-term fertilization experiments. Sci. Total Environ. 2019, 660, 1029–1037. [Google Scholar] [CrossRef]
- Bratina, B.; Šorgo, A.; Kramberger, J.; Ajdnik, U.; Zemljič, L.F.; Ekart, J.; Šafarič, R. From municipal/industrial wastewater sludge and FOG to fertilizer: A proposal for economic sustainable sludge management. J. Environ. Manag. 2016, 183, 1009–1025. [Google Scholar] [CrossRef] [PubMed]
- Wolka, K.; Melaku, B. Exploring selected plant nutrient in compost prepared from food waste and cattle manure and its effect on soil properties and maize yield at Wondo Genet, Ethiopia. Environ. Syst. Res. 2015, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Altieri, R.; Esposito, A. Evaluation of the fertilizing effect of olive mill waste compost in short-term crops. Int. Biodeterior. Biodegrad. 2010, 64, 124–128. [Google Scholar] [CrossRef]
- Jiang, Y.; Ju, M.T.; Li, W.Z.; Ren, Q.B.; Liu, L.; Chen, Y.; Yang, Q.; Hou, Q.D.; Liu, Y.L. Rapid production of organic fertilizer by dynamic high-temperature aerobic fermentation (DHAF) of food waste. Bioresour. Technol. 2015, 197, 7–14. [Google Scholar] [CrossRef]
- Zhao, Q.S.; Li, P.P.; Sun, D.M. Effects of inoculating thermophiles and Rhizopus on composting process of vinegar residue and their nutrients status. Adv. Mat. Res. 2012, 518–523, 68–72. [Google Scholar] [CrossRef]
- Negi, S.; Mandpe, A.; Hussain, A.; Kumar, S. Collegial effect of maggots larvae and garbage enzyme in rapid composting of food waste with wheat straw or biomass waste. J. Clean. Prod. 2020, 258, 120854. [Google Scholar] [CrossRef]
- Rehman, R.A.; Qayyum, M.F. Co-composts of sewage sludge, farm manure and rock phosphate can substitute phosphorus fertilizers in rice-wheat cropping system. J. Environ. Manag. 2020, 259, 109700. [Google Scholar] [CrossRef]
- Ezugworie, F.N.; Igbokwe, V.C.; Onwosi, C.O. Proliferation of antibiotic-resistant microorganisms and associated genes during composting: An overview of the potential impacts on public health, management and future. Sci. Total Environ. 2021, 784, 147191. [Google Scholar] [CrossRef]
- Xi, B.D.; He, X.S.; Dang, Q.L.; Yang, T.X.; Li, M.X.; Wang, X.W.; Li, D.; Tang, J. Effect of multi-stage inoculation on the bacterial and fungal community structure during organic municipal solid wastes composting. Bioresour. Technol. 2015, 196, 399–405. [Google Scholar] [CrossRef]
- Ajmal, M.; Shi, A.; Awais, M.; Mengqi, Z.; Zihao, X.; Shabbir, A.; Faheem, M.; Wei, W.; Ye, L. Ultra-high temperature aerobic fermentation pretreatment composting: Parameters optimization, mechanisms and compost quality assessment. J. Environ. Chem. Eng. 2021, 9, 105453. [Google Scholar] [CrossRef]
- Chang, R.X.; Li, Y.T.; Li, N.H.; Wu, X.H.; Chen, Q. Effect of microbial transformation induced by metallic compound additives and temperature variations during composting on suppression of soil-borne pathogens. J. Environ. Manag. 2021, 279, 111816. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.Q.; Li, W.G.; Zhang, S.M.; Wu, C.D.; Lv, L.Y. Feasibility of co-composting of sewage sludge, spent mushroom substrate and wheat straw. Bioresour. Technol. 2017, 226, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Kumar Awasthi, M.; Wu, L.L.; Yan, Y.; Lv, J.L. Microbial driving mechanism of biochar and bean dregs on NH3 and N2O emissions during composting. Bioresour. Technol. 2020, 315, 123829. [Google Scholar] [CrossRef]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sanchez, A. Recycling of organic wastes through composting: Process performance and compost application in agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Heinze, T.; Philipp, B.; Wagenknecht, W. New approaches to advanced polymers by selective cellulose functionalization. Acta Polym. 1997, 48, 277–297. [Google Scholar] [CrossRef]
- Koschella, A.; Fenn, D.; Illy, N.; Heinze, T. Regioselectively functionalized cellulose derivatives: A mini review. Macromol. Symp. 2006, 244, 59–73. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Li, Z.Y.; Lu, X.N.; Duan, Q.N.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- He, B.; Yun, Z.J.; Shi, J.B.; Jiang, G.B. Research progress of heavy metal pollution in China: Sources, analytical methods, status, and toxicity. Chin. Sci. Bull. 2013, 58, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Forjan, R.; Asensio, V.; Rodriguez-Vila, A.; Covelo, E.F. Contribution of waste and biochar amendment to the sorption of metals in a copper mine tailing. CATENA 2016, 137, 120–125. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Ali, G.A.M.; Bashir, M.J.K.; Copty, N.K.; Abu Amr, S.S.; Abushammala, M.F.M.; Al Maskari, T. Recent advances of nanoremediation technologies for soil and groundwater remediation: A review. Water 2021, 13, 2186. [Google Scholar] [CrossRef]
- Gao, Y.N.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.L.; Gao, B.; Wang, S.S.; Wang, B. Biochar as a potential strategy for remediation of contaminated mining soils: Mechanisms, applications, and future perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.; Aghbashlo, M.; Valijanian, E.; Panahi, H.K.S.; Nizami, A.S.; Ghanavati, H.; Sulaiman, A.; Mirmohamadsadeghi, S.; Karimi, K. A comprehensive review on recent biological innovations to improve biogas production, Part 1: Upstream strategies. Renew. Energ. 2020, 146, 1204–1220. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Valijanian, E.; Panahi, H.K.S.; Nizami, A.S.; Ghanavati, H.; Sulaiman, A.; Mirmohamadsadeghi, S.; Karimi, K. A comprehensive review on recent biological innovations to improve biogas production, Part 2: Mainstream and downstream strategies. Renew. Energ. 2020, 146, 1392–1407. [Google Scholar] [CrossRef]
- Casals, E.; Barrena, R.; Garcia, A.; Gonzalez, E.; Delgado, L.; Busquets-Fite, M.; Font, X.; Arbiol, J.; Glatzel, P.; Kvashnina, K.; et al. Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small 2014, 10, 2801–2808. [Google Scholar] [CrossRef] [Green Version]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Effects of Co and Ni nanoparticles on biogas and methane production from anaerobic digestion of slurry. Energ. Convers. Manage. 2017, 141, 108–119. [Google Scholar] [CrossRef]
- Treu, L.; Campanaro, S.; Kougias, P.G.; Zhu, X.Y.; Angelidaki, I. Untangling the effect of fatty acid addition at species level revealed different transcriptional responses of the biogas microbial community members. Environ. Sci. Technol. 2016, 50, 6079–6090. [Google Scholar] [CrossRef] [Green Version]
- Xia, A.; Cheng, J.; Murphy, J.D. Innovation in biological production and upgrading of methane and hydrogen for use as gaseous transport biofuel. Biotechnol. Adv. 2016, 34, 451–472. [Google Scholar] [CrossRef]
- Onwosi, C.O.; Igbokwe, V.C.; Odimba, J.N.; Eke, I.E.; Nwankwoala, M.O.; Iroh, I.N.; Ezeogu, L.I. Composting technology in waste stabilization: On the methods, challenges and future prospects. J. Environ. Manag. 2017, 190, 140–157. [Google Scholar] [CrossRef]
- Guo, H.N.; Wu, S.B.; Tian, Y.J.; Zhang, J.; Liu, H.T. Application of machine learning methods for the prediction of organic solid waste treatment and recycling processes: A review. Bioresour. Technol. 2021, 319, 124114. [Google Scholar] [CrossRef]






| Items | Unit | Value |
|---|---|---|
| Total solid | % | 27.53–35.63 |
| VS/TS | % | 88.96–94.73 |
| Crude protein | %TS | 9.56–17.60 |
| Crude fiber | %TS | 26.70–34.92 |
| Crude fat | %TS | 2.70–6.53 |
| Residual starch | k/kg TS | 16.12–16.32 |
| Hemicellulose | %TS | 16.22–38.90 |
| Cellulose | %TS | 22.96–34.91 |
| Lignin | %TS | 9.20–24.78 |
| Neutral detergent fiber | %TS | 62.40–85.17 |
| Acid detergent fiber | %TS | 46.89–55.13 |
| Acid detergent lignin | %TS | 22.74–22.74 |
| Ether extract | %TS | 5.95–9.98 |
| Acetic acid | k/kg TS | 0.15–1.02 |
| Lactic acid | k/kg TS | 0.12–1.10 |
| Tartaric acid | k/kg TS | 0.16–0.19 |
| Malic acid | k/kg TS | 0.04–0.08 |
| Ash | %TS | 5.62–13.17 |
| C | %TS | 42.14–49.12 |
| N | %TS | 1.68–6.61 |
| C/N | NA | 15.50–28.68 |
| H | %TS | 4.88–6.83 |
| S | %TS | 0.08–0.38 |
| O | %TS | 35.25–43.44 |
| Calcium | k/kg TS | 2.10–2.50 |
| Phosphorus | k/kg TS | 0.40–0.63 |
| pH | NA | 3.49–4.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Yu, Y.; Liu, S.; Zhu, Y.; Liu, P.; Yu, Z.; Wang, Y. A Review of the Current State and Future Prospects in Resource Recovery of Chinese Cereal Vinegar Residue. Foods 2022, 11, 3256. https://doi.org/10.3390/foods11203256
Wang K, Yu Y, Liu S, Zhu Y, Liu P, Yu Z, Wang Y. A Review of the Current State and Future Prospects in Resource Recovery of Chinese Cereal Vinegar Residue. Foods. 2022; 11(20):3256. https://doi.org/10.3390/foods11203256
Chicago/Turabian StyleWang, Ke, Yongjian Yu, Shuangping Liu, Yuanyuan Zhu, Peng Liu, Zhen Yu, and Yuqin Wang. 2022. "A Review of the Current State and Future Prospects in Resource Recovery of Chinese Cereal Vinegar Residue" Foods 11, no. 20: 3256. https://doi.org/10.3390/foods11203256
APA StyleWang, K., Yu, Y., Liu, S., Zhu, Y., Liu, P., Yu, Z., & Wang, Y. (2022). A Review of the Current State and Future Prospects in Resource Recovery of Chinese Cereal Vinegar Residue. Foods, 11(20), 3256. https://doi.org/10.3390/foods11203256