The Sustainability of Sweet Potato Residues from Starch Processing By-Products: Preparation with Lacticaseibacillus rhamnosus and Pediococcus pentosaceus, Characterization, and Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
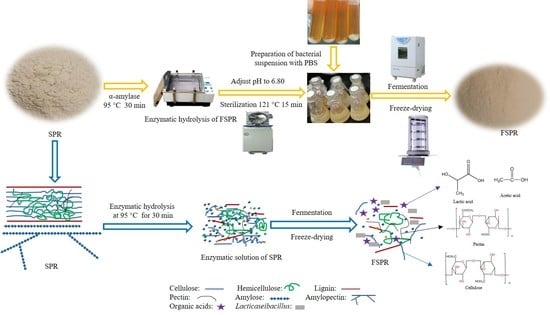
2.2. Investigation of Optimum Process Conditions of Fermented SPR
2.3. Analysis of Nutritional Composition
2.4. Total Polyphenols Content (TPC) and Antioxidant Activities
2.5. pH Value, Total Titratable Acid (TTA) and Organic Acids
2.6. GC-MS Analysis
2.7. Microstructure Analysis
2.7.1. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.7.2. Scanning Electron Microscopy (SEM) Analysis
2.8. In Vitro Saliva–Gastrointestinal Digestion and Colonic Fermentation
2.8.1. In Vitro Saliva–Gastrointestinal Digestion
2.8.2. In Vitro Colonic Fermentation
2.8.3. Gut Microbiota Analysis
2.8.4. Extraction and Characterization of TDF from FSPR during Different In Vitro Stages
2.9. Statistical Analysis
3. Results
3.1. Effects of Fermentation Parameters on the Organic Acid Contents and Viable Bacteria Count of Fermented SPR
3.2. Chemical Characterization of FSPR under Optimal Process Conditions
3.2.1. Nutritional–Functional Compositions
3.2.2. TPC and Antioxidant Activities
3.2.3. pH Value, TTA, and Organic Acids
3.2.4. GC-MS analysis
3.2.5. Structural Analysis of FSPR
FTIR
SEM
3.3. Viable Bacteria Count and TPC of FSPR during In Vitro Digestion and Colonic Fermentation
3.4. The Value of Total Acids, pH Value, Lactic Acid and SCFAs during Simulated Colonic Fermentation
3.5. The Effects of FSPR on Gut Microbiota Compositions during Simulated Colonic Fermentation
3.6. Characterization of TDF from FSPR during In Vitro Digestion and Colonic Fermentation
3.6.1. FTIR
3.6.2. XRD
3.6.3. SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.L.; Mu, T.H.; Ma, M.M.; Sun, H.N.; Zhao, G.H. Nutritional composition, antioxidant activity, volatile compounds, and stability properties of sweet potato residues fermented with selected lactic acid bacteria and bifidobacteria. Food Chem. 2022, 374, 131500. [Google Scholar] [CrossRef] [PubMed]
- Ogutu, F.O.; Mu, T.H.; Sun, H.N.; Zhang, M. Ultrasonic Modified Sweet Potato Pectin Induces Apoptosis like Cell Death in Colon Cancer (HT-29) Cell Line. Nutr. Cancer 2018, 70, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.Z.; Zhou, S.M.; Wang, T.T.Y.; Zhou, S.H.; Yang, K.L.; Li, Y.X.; Tian, J.; Wang, J. Dietary fiber isolated from sweet potato residues promotes a healthy gut microbiome profile. Food Funct. 2020, 11, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.H.; Zhang, F.; Chai, Z.Y.; Liu, M.; Battino, M.; Meng, X.H. Mixed fermentation of blueberry pomace with L. rhamnosus GG and L. plantarum-1: Enhance the active ingredient, antioxidant activity and health-promoting benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.J.; Tao, Y.; Li, D.D.; Han, Y.B.; Show, P.L.; Wen, G.Z.; Zhou, J.Z. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Li, T.L.; Jiang, T.; Liu, N.; Wu, C.Y.; Xu, H.D.; Lei, H.J. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.M.; Zhang, L. Optimization of lactic acid fermentation conditions for fermented tofu whey beverage with high-isoflavone aglycones. Lwt. Food Sci. Technol. 2019, 111, 211–217. [Google Scholar] [CrossRef]
- Bujna, E.; Farkas, N.A.; Tran, A.M.; Dam, M.S.; Nguyen, Q.D. Lactic acid fermentation of apricot juice by mono- and mixed cultures of probiotic Lactobacillus and Bifidobacterium strains. Food Sci. Biotechnol. 2018, 27, 547–554. [Google Scholar] [CrossRef]
- Yan, J.; Xue, Q.Y.; Chen, W.Y.; Wang, K.; Peng, D.; Jiang, J.J.; Li, P.; Du, B. Probiotic-fermented rice buckwheat alleviates high-fat diet-induced hyperlipidemia in mice by suppressing lipid accumulation and modulating gut microbiota. Food Res. Int. 2022, 155, 111125. [Google Scholar] [CrossRef]
- Likotrafiti, E.; Tuohy, K.M.; Gibson, G.R.; Rastall, R.A. An in vitro study of the effect of probiotics, prebiotics and synbiotics on the elderly faecal microbiota. Anaerobe 2014, 27, 50–55. [Google Scholar] [CrossRef]
- Duedu, K.O.; French, C.E. Data for discriminating dead/live bacteria in homogenous cell suspensions and the effect of insoluble substrates on turbidimetric measurements. Data Brief. 2017, 12, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahlany, S.T.; Niamah, A.K. Bacterial viability, antioxidant stability, antimutagenicity and sensory properties of onion types fermentation by using probiotic starter during storage. Nutr Food Sci. 2022, 52, 901–916. [Google Scholar] [CrossRef]
- Ma, M.M.; Mu, T.H. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Mu, T.H.; Zhang, M.; Ma, M.M. Effects of different polysaccharides and proteins on dough rheological properties, texture, structure and in vitro starch digestibility of wet sweet potato vermicelli. Int. J. Biol. Macromol. 2020, 148, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.T.; Yan, Y.M.; Mi, J.; Zhang, H.C.; Lu, L.; Luo, Q.; Li, X.Y.; Zeng, X.X.; Cao, Y.L. Simulated Digestion and Fermentation in Vitro by Human Gut Microbiota of Polysaccharides from Bee Collected Pollen of Chinese Wolfberry. J. Agric. Food Chem. 2018, 66, 898–907. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Bi, J.F.; Yi, J.Y.; Wu, X.Y.; Ma, Y.C.; Li, R.P. Pectin and homogalacturonan with small molecular mass modulate microbial community and generate high SCFAs via in vitro gut fermentation. Carbohyd. Polym. 2021, 269, 118326. [Google Scholar] [CrossRef] [PubMed]
- Karbasi, M.; Yarmand, M.S.; Mousavi, M. Fermentation Potential of Lactobacillus rhamnosus and Lactobacillus acidophilus in Date Syrup to Develop a Functional Fermented Beverage: A Comparative Study. J. Food Process. Pres. 2015, 39, 863–870. [Google Scholar] [CrossRef]
- Sharma, A.; Mukherjee, S.; Reddy Tadi, S.R.; Ramesh, A.; Sivaprakasam, S. Kinetics of growth, plantaricin and lactic acid production in whey permeate based medium by probiotic Lactobacillus plantarum CRA52. LWT-Food Sci. Technol. 2021, 139, 110744. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Process optimization for the development of a functional beverage based on lactic acid fermentation of oats. Biochem. Eng. J. 2010, 52, 199–204. [Google Scholar] [CrossRef]
- Mustafa, S.M.; Chua, L.S.; El-Enshasy, H.A.; Majid, F.A.A.; Hanapi, S.Z.; Malik, R.A. Effect of temperature and pH on the probiotication of Punica granatum juice using Lactobacillus species. J. Food Biochem. 2019, 43, e12805. [Google Scholar] [CrossRef]
- Wu, W.J.; Hu, J.; Gao, H.Y.; Chen, H.J.; Fang, X.J.; Mu, H.L.; Han, Y.C.; Liu, R.L. The potential cholesterol-lowering and prebiotic effects of bamboo shoot dietary fibers and their structural characteristics. Food Chem. 2020, 332, 127372. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, T.M.R.; Sampaio, K.B.; Souza, E.L. Sweet potato roots: Unrevealing an old food as a source of health promoting bioactive compounds—A review. Trends Food Sci. Technol. 2019, 85, 277–286. [Google Scholar] [CrossRef]
- Ju, D.; Mu, T.H.; Sun, H.N. Sweet potato and potato residual flours as potential nutritional and healthy food material. J. Integr. Agric. 2017, 16, 2632–2645. [Google Scholar] [CrossRef] [Green Version]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Jayabalan, R. Effect of probiotification with Lactobacillus plantarum MCC 2974 on quality of Sohiong juice. Lwt-Food Sci Technol. 2019, 108, 55–60. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Wang, Y.B.; Yang, G.; Zhang, Q.H.; Meng, L.B.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Huang, J.T.; Wang, Y.Q.; Wang, X.N.; Ren, Y.C.; Yue, T.L.; Wang, Z.L.; Gao, Z.P. Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem. 2021, 363, 130351. [Google Scholar] [CrossRef]
- Liu, S.; Jia, M.Y.; Chen, J.J.; Wan, H.S.; Dong, R.H.; Nie, S.P.; Xie, M.Y.; Yu, Q. Removal of bound polyphenols and its effect on antioxidant and prebiotics properties of carrot dietary fiber. Food Hydrocolloid. 2019, 93, 284–292. [Google Scholar] [CrossRef]
- Bader, U.A.H.; Saeed, F.; Khan, A.M.; Niaz, B.; Rohi, M.; Nasir, M.A.; Tufail, T.; Anbreen, F.; Anjum, F.M. Modification of barley dietary fiber through thermal treatments. Food Sci. Nutr. 2019, 7, 1816–1820. [Google Scholar] [CrossRef] [Green Version]
- García-Hernández, H.J.; Hernández-Pérez, M.; Peinado, I.; Andrés, A.; Heredia, A. Tomato-antioxidants enhance viability of L. reuteri under gastrointestinal conditions while the probiotic negatively affects bioaccessibility of lycopene and phenols. J. Funct. Foods 2018, 43, 1–7. [Google Scholar] [CrossRef]
- Costa, M.G.M.; Ooki, G.N.; Vieira, A.D.S.; Bedani, R.; Saad, S.M.I. Synbiotic Amazonian palm berry (acai, Euterpe oleracea Mart.) ice cream improved Lactobacillus rhamnosus GG survival to simulated gastrointestinal stress. Food Funct. 2017, 8, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.H.; Liu, S.; Xie, J.H.; Chen, Y.; Zheng, Y.T.; Zhang, X.J.; Zhao, E.; Wang, Z.P.; Xu, H.Y.; Yu, Q. The recovery, catabolism and potential bioactivity of polyphenols from carrot subjected to in vitro simulated digestion and colonic fermentation. Food Res. Int. 2021, 143, 110263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Zhang, M.W.; Dong, L.H.; Jia, X.C.; Liu, L.; Ma, Y.X.; Huang, F.; Zhang, R.F. Phytochemical Profile, Bioactivity, and Prebiotic Potential of Bound Phenolics Released from Rice Bran Dietary Fiber during in Vitro Gastrointestinal Digestion and Colonic Fermentation. J. Agric. Food Chem. 2019, 67, 12796–12805. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Yuan, Q.; Guo, H.; Fu, Y.; Li, F.; Wang, S.P.; Gan, R.Y. Dynamic changes of structural characteristics of snow chrysanthemum polysaccharides during in vitro digestion and fecal fermentation and related impacts on gut microbiota. Food Res. Int. 2021, 141, 109888. [Google Scholar] [CrossRef]
- Zhang, X.; Aweya, J.J.; Huang, Z.X.; Kang, Z.Y.; Bai, Z.H.; Li, K.H.; He, X.T.; Liu, Y.; Chen, X.Q.; Cheong, K. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohyd. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef]
- Tang, S.X.; Cheng, Y.X.; Wu, T.; Hu, F.T.; Pan, S.Y.; Xu, X.Y. Effect of Lactobacillus plantarum-fermented mulberry pomace on antioxidant properties and fecal microbial community. LWT Food Sci. Technol. 2021, 147, 111651. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Chen, J.; Chen, F.L.; Wang, X.C.; Zhu, Q.J.; Ao, Q. Surface characterization of corn stalk superfine powder studied by FTIR and XRD. Colloid. Surface B 2013, 104, 207–212. [Google Scholar] [CrossRef]
- Cantu-Jungles, J.T.M.; Zhang, X.W.; Kazem, A.E.; Iacomini, M.; Hamaker, B.R.; Cordeiro, L.M.C. Microwave treatment enhances human gut microbiota fermentability of isolated insoluble dietary fibers. Food Res. Int. 2021, 143, 110293. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.X.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404. [Google Scholar] [CrossRef]






| Index | SPR | FSPR |
|---|---|---|
| Total starch | 51.01 ± 2.65 | 3.40 ± 0.40 |
| Reducing sugars | 2.50 ± 0.13 | 18.02 ± 0.81 |
| Insoluble dietary fiber | 25.09 ± 3.61 | 20.59 ± 2.81 |
| Soluble dietary fiber | 10.17 ± 0.95 | 15.02 ± 0.58 |
| Total dietary fiber | 35.26 ± 2.85 | 35.61 ± 2.97 |
| Protein | 3.95 ± 0.09 | 3.88 ± 0.08 |
| Soluble protein | 475.74 ± 12.42 | 141.53 ± 5.76 |
| Ash | 2.41 ± 0.04 | 3.29 ± 0.12 |
| pH | 5.48 ± 0.12 | 3.26 ± 0.08 |
| Titratable acid | 1.06 ± 0.00 | 6.86 ± 0.63 |
| Lactic acid | 0.05 ± 0.01 | 58.01 ± 1.42 |
| Acetic acid | 0.10 ± 0.01 | 1.66 ± 0.20 |
| Total polyphenol contents | 70.47 ± 10.89 | 95.74 ± 1.20 |
| ABTS | 12.70 ± 0.57 | 13.70 ± 0.85 |
| DPPH | 33.92 ± 1.90 | 57.07 ± 1.24 |
| FRAP | 27.18 ± 1.36 | 60.51 ± 1.03 |
| Volatile Compounds | Area% of Each Sample | |
|---|---|---|
| SPR | FSPR | |
| Acids | ND | 34.26±6.95 |
| Decanedioic acid | ND | 1.46 ± 0.46 |
| Sorbic Acid | ND | 28.68 ± 5.57 |
| Acetic acid | ND | 2.60 ± 0.76 |
| Hexanoic acid | ND | 1.52 ± 0.16 |
| Aldehydes | 68.08 ± 4.58 | 26.33 ± 1.83 |
| Nonanal | 14.63 ± 1.14 | 10.98 ± 1.35 |
| Decanal | 9.60 ± 1.53 | 6.30 ± 1.11 |
| Benzaldehyde | 4.04 ± 1.01 | 1.70 ± 1.32 |
| (E)-2-Nonenal | 35.61 ± 0.14 | 6.84 ± 0.44 |
| Benzaldehyde, 4-(1- | ||
| methylethyl)- | 2.90 ± 0.51 | 0.51 ± 0.04 |
| (E, E)-2,4-Nonadienal | 1.30 ± 0.25 | ND |
| Alcohols | ND | 1.33 ± 0.26 |
| Geraniol | ND | 1.34 ± 0.12 |
| Phenylethyl Alcohol | ND | 0.56 ± 0.06 |
| 3,5-Dimethyl-Cyclohexanol | ND | 0.77 ± 0.08 |
| Esters | 1.60 ± 0.24 | 1.88 ± 0.09 |
| Hexadecanoic acid methyl ester | 1.60 ± 0.24 | 1.88 ± 0.09 |
| Ketones | 1.68 ± 0.43 | 6.08 ± 1.09 |
| 3-Hydroxybutanone | ND | 1.59 ± 0.53 |
| 2-Nonanone | 1.68 ± 0.43 | 2.43 ± 0.05 |
| 2-Undecanone | ND | 0.82 ± 0.16 |
| Dihydro-5-pentyl-2(3H)-Furanone | ND | 2.83 ± 0.35 |
| Others | 22.52 ± 3.32 | 19.09 ± 2.31 |
| 2-pentyl-Furan | 10.16 ± 1.51 | 12.19 ± 0.53 |
| Heptadecane | 5.45 ± 0.3 | ND |
| Dodecane | ND | 3.08 ± 1.54 |
| Eicosane | 3.87 ± 1.05 | 1.55 ± 0.04 |
| 1-methyl-Naphthalene | 3.04 ± 0.46 | 0.61 ± 0.05 |
| Butylated Hydroxytoluene | ND | 1.66 ± 0.15 |
| Ace | Chao | Shannon | Simpson | |
|---|---|---|---|---|
| CK12 | 390.45 ± 13.81 a | 386.53 ± 17.94 b | 3.99 ± 0.03 fg | 0.04 ± 0.00 a |
| CK24 | 384.88 ± 8.20 bc | 379.98 ± 5.83 b | 4.06 ± 0.01 g | 0.03 ± 0.00 a |
| CK48 | 394.31 ± 11.25 c | 395.69 ± 17.81 b | 3.90 ± 0.06 ef | 0.04 ± 0.00 a |
| FSPR12 | 377.92 ± 8.16 bc | 377.13 ± 5.66 b | 3.39 ± 0.05 ef | 0.12 ± 0.01 a |
| FSPR24 | 375.21 ± 20.8 bc | 385.16 ± 15.19 b | 3.28 ± 0.08 bc | 0.13 ± 0.02 d |
| FSPR48 | 332.01 ± 43.47 ab | 363.08 ± 36.37 b | 3.27 ± 0.14 bc | 0.09 ± 0.01 c |
| SPR12 | 387.48 ± 9.20 bc | 383.50 ± 8.44 b | 3.55 ± 0.15 d | 0.08 ± 0.03 c |
| SPR24 | 381.06 ± 29.71 bc | 382.49 ± 30.75 b | 3.22 ± 0.04 b | 0.13 ± 0.01 d |
| SPR48 | 320.21 ± 42.35 a | 311.53 ± 40.63 a | 3.01 ± 0.11 a | 0.12 ± 0.02 d |
| FOS12 | 362.36 ± 9.21 abc | 365.92 ± 16.49 b | 3.89 ± 0.05 ef | 0.05 ± 0.00 ab |
| FOS24 | 386.42 ± 20.96 bc | 387.90 ± 21.59 b | 3.79 ± 0.03 e | 0.06 ± 0.00 b |
| FOS48 | 395.60 ± 63.37 c | 394.62 ± 37.72 b | 3.41 ± 0.04 c | 0.10 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Sun, H.; Ma, M.; Mu, T.; Zhao, G.; Lwin, M.M. The Sustainability of Sweet Potato Residues from Starch Processing By-Products: Preparation with Lacticaseibacillus rhamnosus and Pediococcus pentosaceus, Characterization, and Application. Foods 2023, 12, 128. https://doi.org/10.3390/foods12010128
Zhu L, Sun H, Ma M, Mu T, Zhao G, Lwin MM. The Sustainability of Sweet Potato Residues from Starch Processing By-Products: Preparation with Lacticaseibacillus rhamnosus and Pediococcus pentosaceus, Characterization, and Application. Foods. 2023; 12(1):128. https://doi.org/10.3390/foods12010128
Chicago/Turabian StyleZhu, Lili, Hongnan Sun, Mengmei Ma, Taihua Mu, Guohua Zhao, and Moe Moe Lwin. 2023. "The Sustainability of Sweet Potato Residues from Starch Processing By-Products: Preparation with Lacticaseibacillus rhamnosus and Pediococcus pentosaceus, Characterization, and Application" Foods 12, no. 1: 128. https://doi.org/10.3390/foods12010128
APA StyleZhu, L., Sun, H., Ma, M., Mu, T., Zhao, G., & Lwin, M. M. (2023). The Sustainability of Sweet Potato Residues from Starch Processing By-Products: Preparation with Lacticaseibacillus rhamnosus and Pediococcus pentosaceus, Characterization, and Application. Foods, 12(1), 128. https://doi.org/10.3390/foods12010128