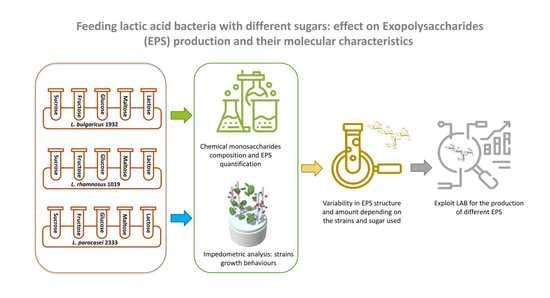
Feeding Lactic Acid Bacteria with Different Sugars: Effect on Exopolysaccharides (EPS) Production and Their Molecular Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Growth Conditions and Media
2.2. Growth Behaviour of the Strains with Different Sugars
2.3. Impedance Measurement for the Detection of EPS Production
2.4. EPS Extraction and Quantification
2.5. EPS Monosaccharide Composition by Gas Chromatography-Mass Spectrometry (GC-MS)
2.6. Evaluation of the EPS Molecular Weight by HPSEC-RID
2.7. Statistical Analysis
3. Results and Discussion
3.1. Growth Behaviour of the Strains with Different Sugars
3.2. Quantification of EPS Production
3.3. EPS Monosaccharide Composition
3.4. EPS Molecular Weight (Mw)
3.5. Correlation Analysis between Factors Involved in EPS Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Mozzi, F.; Gerbino, E.; de Valdez, G.F.; Torino, M.I. Functionality of exopolysaccharides produced by lactic acid bacteria in an in vitro gastric system. J. Appl. Microbiol. 2009, 107, 56–64. [Google Scholar] [CrossRef]
- Dertli, E.; Mercan, E.; Arıcı, M.; Yılmaz, M.T.; Sağdıç, O. Characterisation of lactic acid bacteria from Turkish sourdough and determination of their exopolysaccharide (EPS) production characteristics. LWT-Food Sci. Technol. 2016, 71, 116–124. [Google Scholar] [CrossRef]
- Nachtigall, C.; Surber, G.; Herbi, F.; Wefers, D.; Jaros, D.; Rohm, H. Production and molecular structure of heteropolysaccharides from two lactic acid bacteria. Carbohydr. Polym. 2020, 236, 116019. [Google Scholar] [CrossRef]
- Ispirli, H.; Demirbaş, F.; Dertli, E. Glucan type exopolysaccharide (EPS) shows prebiotic effect and reduces syneresis in chocolate pudding. J. Food Sci. Technol. 2018, 55, 3821–3826. [Google Scholar] [CrossRef]
- Tang, W.; Zhou, J.; Xu, Q.; Dong, M.; Fan, X.; Rui, X.; Zhang, Q.; Chen, X.; Jiang, M.; Wu, J.; et al. In vitro digestion and fermentation of released exopolysaccharides (r-EPS) from Lactobacillus delbrueckii ssp. bulgaricus SRFM-1. Carbohydr. Polym. 2020, 230, 115593. [Google Scholar] [CrossRef] [PubMed]
- Korcz, E.; Kerényi, Z.; Varga, L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef]
- Abarquero, D.; Renes, E.; Fresno, J.M.; Tornadijo, M.E. Study of exopolysaccharides from lactic acid bacteria and their industrial applications: A review. Int. J. Food Sci. Technol. 2022, 57, 16–26. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Pan, L.; Han, Y.; Zhou, Z. In vitro prebiotic activities of exopolysaccharide from Leuconostoc pseudomesenteroides XG5 and its effect on the gut microbiota of mice. J. Funct. Foods 2020, 67, 103853. [Google Scholar] [CrossRef]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of health benefits conferred by Lactobacillus species from kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Xu, D.; Tang, N.; Rui, X.; Zhang, Q.; Chen, X.; Dong, M.; Li, W. Biosynthesis of exopolysaccharide and structural characterization by Lacticaseibacillus paracasei ZY-1 isolated from Tibetan kefir. Food Chem. Mol. Sci. 2021, 3, 100054. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.F.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef] [Green Version]
- Nowak, B.; Śróttek, M.; Ciszek-Lenda, M.; Skałkowska, A.; Gamian, A.; Górska, S.; Marcinkiewicz, J. Exopolysaccharide from Lactobacillus rhamnosus KL37 Inhibits T Cell-dependent Immune Response in Mice. Arch. Immunol. Ther. Exp. 2020, 68, 17. [Google Scholar] [CrossRef]
- Li, B.; Du, P.; Smith, E.E.; Wang, S.; Jiao, Y.; Guo, L.; Huo, G.; Liu, F. In vitro and in vivo evaluation of an exopolysaccharide produced by Lactobacillus helveticus KLDS1.8701 for the alleviative effect on oxidative stress. Food Funct. 2019, 10, 1707–1717. [Google Scholar] [CrossRef]
- Wang, B.; Song, Q.; Zhao, F.; Han, Y.; Zhou, Z. Production optimization, partial characterization and properties of an exopolysaccharide from Lactobacillus sakei L3. Int. J. Biol. Macromol. 2019, 141, 21–28. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Wang, X.; Yue, F.; Shan, Y.; Liu, B.; Zhou, Y.; Yi, Y.; Lü, X. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2019, 128, 480–492. [Google Scholar] [CrossRef]
- Sahin, A.W.; Zannini, E.; Coffey, A.; Arendt, E.K. Sugar reduction in bakery products: Current strategies and sourdough technology as a potential novel approach. Food Res. Int. 2019, 126, 108583. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Galle, S.; Gänzle, M.G.; Waters, D.M.; Arendt, E.K. Evaluation of exopolysaccharide producing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol. 2014, 37, 44–50. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Degeest, B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Polak-Berecka, M.; Waśko, A.; Szwajgier, D.; Choma, A. Bifidogenic and antioxidant activity of exopolysaccharides produced by Lactobacillus rhamnosus E/N cultivated on different carbon sources. Pol. J. Microbiol. 2013, 62, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Mıdık, F.; Tokatlı, M.; Elmacı, S.B.; Özçelik, F. Influence of different culture conditions on exopolysaccharide production by indigenous lactic acid bacteria isolated from pickles. Arch. Microbiol. 2020, 202, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Huang, L.; Li, K.-T. Antioxidant activity changes of exopolysaccharides with different carbon sources from Lactobacillus plantarum LPC-1 and its metabolomic analysis. World J. Microbiol. Biotechnol. 2019, 35, 68. [Google Scholar] [CrossRef]
- Hebert, E.M.; Raya, R.R.; Brown, L.; de Valdez, G.F.; de Giori, G.S.; Taranto, M.P. Genome sequence of the cheese-starter strain Lactobacillus delbrueckii subsp. lactis CRL 581. Genome Announc. 2013, 1, e00602-13. [Google Scholar] [CrossRef] [Green Version]
- Vinderola, C.G.; Costa, G.A.; Regenhardt, S.; Reinheimer, J.A. Influence of compounds associated with fermented dairy products on the growth of lactic acid starter and probiotic bacteria. Int. Dairy J. 2002, 12, 579–589. [Google Scholar] [CrossRef]
- Bottari, B.; Felis, G.E.; Salvetti, E.; Castioni, A.; Campedelli, I.; Torriani, S.; Bernini, V.; Gatti, M. Effective identification of Lactobacillus casei group species: Genome-based selection of the gene mutL as the target of a novel multiplex PCR assay. Microbiology 2017, 163, 950–960. [Google Scholar] [CrossRef]
- Degeest, B.; Mozzi, F.; De Vuyst, L. Effect of medium composition and temperature and pH changes on exopolysaccharide yields and stability during Streptococcus thermophilus LY03 fermentations. Int. J. Food Microbiol. 2002, 79, 161–174. [Google Scholar] [CrossRef]
- Degeest, B.; De Vuyst, L. Indication that the nitrogen source influences both amount and size of exopolysaccharides produced by Streptococcus thermophilus LY03 and modelling of the bacterial growth and exopolysaccharide production in a complex medium. Appl. Environ. Microbiol. 1999, 65, 2863–2870. [Google Scholar] [CrossRef]
- Bancalari, E.; Bernini, V.; Bottari, B.; Neviani, E.; Gatti, M. Application of impedance microbiology for evaluating potential acidifying performances of starter lactic acid bacteria to employ in milk transformation. Front. Microbiol. 2016, 7, 1628. [Google Scholar] [CrossRef] [Green Version]
- Bancalari, E.; D’Incecco, P.; Savo Sardaro, M.L.; Neviani, E.; Pellegrino, L.; Gatti, M. Impedance microbiology to speed up the screening of lactic acid bacteria exopolysaccharide production. Int. J. Food Microbiol. 2019, 306, 108268. [Google Scholar] [CrossRef] [PubMed]
- Bancalari, E.; Castellone, V.; Bottari, B.; Gatti, M. Wild Lactobacillus casei Group Strains: Potentiality to ferment plant derived juices. Foods 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.C.; Prosky, L.; De Vries, J.W. Determination of Total, Soluble, and Insoluble Dietary Fiber in Foods—Enzymatic-Gravimetric Method, MES-TRIS Buffer: Collaborative Study. J. AOAC Int. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- Xia, Y.-G.; Wang, T.-L.; Sun, H.-M.; Liang, J.; Kuang, H.-X. Gas chromatography–mass spectrometry-based trimethylsilyl-alditol derivatives for quantitation and fingerprint analysis of Anemarrhena asphodeloides Bunge polysaccharides. Carbohydr. Polym. 2018, 198, 155–163. [Google Scholar] [CrossRef]
- McCleary, B.V.; DeVries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Champ, M.; Okuma, K. Determination of insoluble, soluble, and total dietary fiber (CODEX definition) by enzymatic-gravimetric method and liquid chromatography: Collaborative study. J. AOAC Int. 2012, 95, 824–844. [Google Scholar] [CrossRef]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D. Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef] [Green Version]
- Tayuan, C.; Tannock, G.W.; Rodtong, S. Growth and exopolysaccharide production by Weissella sp. from low-cost substitutes for sucrose. Afr. J. Microbiol. Res. 2011, 5, 3693–3701. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, X.; Jin, H.; Man, C.; Jiang, Y. The effect of optimized carbon source on the synthesis and composition of exopolysaccharides produced by Lactobacillus paracasei. J. Dairy Sci. 2021, 104, 4023–4032. [Google Scholar] [CrossRef]
- Joulak, I.; Finore, I.; Nicolaus, B.; Leone, L.; Moriello, A.S.; Attia, H.; Poli, A.; Azabou, S. Evaluation of the production of exopolysaccharides by newly isolated Halomonas strains from Tunisian hypersaline environments. Int. J. Biol. Macromol. 2019, 138, 658–666. [Google Scholar] [CrossRef]
- Vaningelgem, F.; Zamfir, M.; Mozzi, F.; Adriany, T.; Vancanneyt, M.; Swings, J.; De Vuyst, L. Biodiversity of Exopolysaccharides Produced by Streptococcus thermophilus Strains Is Reflected in Their Production and Their Molecular and Functional Characteristics. Appl. Environ. Microbiol. 2004, 70, 900–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomfim, V.B.; Neto, J.H.P.L.; Leite, K.S.; de Andrade Vieira, É.; Iacomini, M.; Silva, C.M.; dos Santos, K.M.O.; Cardarelli, H.R. Partial characterization and antioxidant activity of exopolysaccharides produced by Lactobacillus plantarum CNPC003. LWT 2020, 127, 109349. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, W.; Mu, W. Recent studies on the biological production of D-mannose. Appl. Microbiol. Biotechnol. 2019, 103, 8753–8761. [Google Scholar] [CrossRef]
- Kansandee, W.; Moonmangmee, D.; Moonmangmee, S.; Itsaranuwat, P. Characterization and Bifidobacterium sp. growth stimulation of exopolysaccharide produced by Enterococcus faecalis EJRM152 isolated from human breast milk. Carbohydr. Polym. 2019, 206, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Pradeepa; Shetty, A.D.; Matthews, K.; Hegde, A.R.; Akshatha, B.; Mathias, A.B.; Mutalik, S.; Vidya, S.M. Multidrug resistant pathogenic bacterial biofilm inhibition by Lactobacillus plantarum exopolysaccharide. Bioact. Carbohydr. Diet. Fibre 2016, 8, 7–14. [Google Scholar] [CrossRef]
- Wang, M.; Bi, J. Modification of characteristics of kefiran by changing the carbon source of Lactobacillus kefiranofaciens. J. Sci. Food Agric. 2008, 88, 763–769. [Google Scholar] [CrossRef]
- Salazar, N.; Prieto, A.; Leal, J.A.; Mayo, B.; Bada-Gancedo, J.C.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Production of exopolysaccharides by Lactobacillus and Bifidobacterium strains of human origin, and metabolic activity of the producing bacteria in milk. J. Dairy Sci. 2009, 92, 4158–4168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamet, M.F.; Piermaria, J.A.; Abraham, A.G. Selection of EPS-producing Lactobacillus strains isolated from kefir grains and rheological characterization of the fermented milks. LWT-Food Sci. Technol. 2015, 63, 129–135. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Choma, A.; Waśko, A.; Górska, S.; Gamian, A.; Cybulska, J. Physicochemical characterization of exopolysaccharides produced by Lactobacillus rhamnosus on various carbon sources. Carbohydr. Polym. 2015, 117, 501–509. [Google Scholar] [CrossRef]
- Torino, M.I.; de Valdez, G.F.; Mozzi, F. Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front. Microbiol. 2015, 6, 834. [Google Scholar] [CrossRef]
- Ziadi, M.; Bouzaiene, T.; M’Hir, S.; Zaafouri, K.; Mokhtar, F.; Hamdi, M.; Boisset-Helbert, C. Evaluation of the efficiency of ethanol precipitation and ultrafiltration on the purification and characteristics of exopolysaccharides produced by three lactic acid bacteria. BioMed Res. Int. 2018, 2018, 1896240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, W.-H.; Fang, X.-B.; Wu, T.; Fang, L.; Liu, C.-L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef] [PubMed]



| Strain | Rate | yEnd | ∆E% |
|---|---|---|---|
| L. paracasei 2333 FRU | 8.84 | 33.46 | 16.95 |
| L. paracasei 2333 MAL | 12.58 | 33.6 | −8.21 |
| L. paracasei 2333 SUC | 11.3 | 40.18 | −5.75 |
| L. paracasei 2333 LAC | 11.25 | 46.7 | 25.19 |
| L. paracasei 2333 GLU | 12.26 | 41.27 | 16.2 |
| L. rhamnosus 1019 FRU | 8.16 | 32.47 | 11.19 |
| L. rhamnosus 1019 MAL | 2.49 | 19.58 | −1.77 |
| L. rhamnosus 1019 SUC | 4.3 | 11.54 | 1.71 |
| L. rhamnosus 1019 LAC | 5.95 | 31.16 | 1.9 |
| L. rhamnosus 1019 GLU | 8.89 | 30.25 | 6.72 |
| L. bulgaricus 1932 FRU | 11,34 | 45.92 | 15.27 |
| L. bulgaricus 1932 MAL | 10.59 | 53.59 | 15.42 |
| L. bulgaricus 1932 SUC | 12.94 | 41.61 | 12.59 |
| L. bulgaricus 1932 LAC | 10.36 | 47.66 | 12.79 |
| L. bulgaricus 1932 GLU | 11.3 | 43.11 | 10.65 |
| % of Total Peak Area | ||||||
|---|---|---|---|---|---|---|
| Strain | Carbon Source | Fraction 1 (>500 kDa) | Fraction 2 (130–200 kDa) | Fraction 3 (40–65 kDa) | Fraction 4 (10–25 kDa) | Fraction 5 (<10 kDa) |
| L. paracasei 2333 | Fructose | − | 7 | 38 | 56 | − |
| Maltose | − | 5 | 42 | 53 | − | |
| Sucrose | − | 4 | 44 | 40 | 12 | |
| Lactose | − | 6 | 41 | 52 | − | |
| Glucose | − | 9 | 37 | 54 | − | |
| L. rhamnosus 1019 | Fructose | − | 10 | 42 | 47 | − |
| Maltose | − | 6 | 36 | 57 | − | |
| Sucrose | 4 | 10 | 25 | 29 | 32 | |
| Lactose | 5 | 17 | 36 | 42 | − | |
| Glucose | − | 8 | 34 | 57 | − | |
| L. bulgaricus 1932 | Fructose | 16 | 4 | 61 | 19 | − |
| Maltose | − | 11 | 32 | 48 | 9 | |
| Sucrose | 15 | 5 | 47 | 32 | − | |
| Lactose | 18 | 3 | 24 | 20 | 36 | |
| Glucose | 15 | 3 | 29 | 23 | 30 | |
| Correlations | EPS g/L | Ribose | Mannose | Rhamnose | Fructose | Galactose | Glucose | GlucosAmine | Galactosamine | Rate | yEnd | ∆E | Fraction 1 | Fraction 2 | Fraction 3 | Fraction 4 | Fraction 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPS g/L | 1 | −0.382 | −0.180 | −0.032 | −0.346 | 0.230 | 0.503 | 0.076 | 0.126 | 0.122 | 0.05 | 0.05 | 0.145 | 0.151 | 0.229 | 0.084 | −0.369 |
| Ribose | −0.382 | 1 | −0.468 | −0.268 | 0.549 * | 0.464 | −0.218 | −0.340 | −0.191 | 0.497 | 0.484 | 0.095 | 0.555 * | −0.534 * | 0.229 | −0.560 * | 0.294 |
| Mannose | −0.180 | −0.468 | 1 | −0.371 | 0.002 | −0.489 | −0.168 | −0.071 | −0.038 | −0.392 | −0.504 | −0.096 | −0.463 | 0.127 | −0.038 | 0.243 | −0.009 |
| Rhamnose | −0.032 | −0.268 | −0.371 | 1 | −0.286 | −0.088 | −0.395 | 0.279 | −0.022 | −0.427 | −0.169 | −0.085 | −0.155 | 0.440 | −0.368 | 0.193 | −0.003 |
| Fructose | −0.346 | 0.549 * | 0.002 | −0.286 | 1 | −0.084 | −0.388 | −0.325 | −0.291 | 0.328 | 0.337 | 0.115 | 0.152 | −0.210 | 0.672 ** | −0.283 | −0.195 |
| Galactose | 0.230 | 0.464 | −0.489 | −0.088 | −0.084 | 1 | 0.031 | −0.457 | −0.474 | 0.244 | 0.48 | 0.386 | 0.850 ** | −0.311 | −0.081 | −0.726 ** | 0.457 |
| Glucose | 0.503 | −0.218 | −0.168 | −0.395 | −0.388 | 0.031 | 1 | −0.024 | 0.215 | 0.319 | 0.032 | −0.271 | 0.034 | −0.105 | 0.057 | 0.079 | −0.115 |
| Glucosamine | 0.076 | −0.340 | −0.071 | 0.279 | −0.325 | −0.457 | −0.024 | 1 | 0.848 ** | −0.044 | −0.044 | 0.205 | −0.541 * | 0.219 | −0.165 | 0.778 ** | −0.472 |
| Galactosamine | 0.126 | −0.191 | −0.038 | −0.022 | −0.291 | −0.474 | 0.215 | 0.848 ** | 1 | 0.061 | −0.14 | 0.047 | −0.585 * | 0.161 | −0.070 | 0.762 ** | −0.484 |
| Rate | −0.141 | 0.334 | −0.150 | −0.284 | 0.292 | −0.314 | 0.170 | 0.336 | 0.369 | 1 | 0.792 ** | 0.337 | −0.033 | −0.300 | 0.161 | 0.130 | −0.131 |
| yEnd | −0.189 | 0.400 | −0.389 | −0.083 | 0.488 | −0.017 | −0.051 | 0.250 | 0.089 | 0.792 ** | 1 | 0.563 * | 0.140 | −0.225 | 0.258 | 0.003 | −0.190 |
| ∆E | −0.024 | 0.123 | −0.216 | −0.011 | 0.227 | 0.312 | −0.218 | 0.218 | −0.073 | 0.337 | 0.563 * | 1 | 0.309 | −0.024 | 0.047 | −0.147 | −0.030 |
| Fraction1 | 0.145 | 0.555 * | −0.463 | −0.155 | 0.152 | 0.850 ** | 0.034 | −0.541 * | −0.585 * | 0.272 | 0.341 | 0.216 | 1 | −0.431 | 0.069 | −0.893 ** | 0.483 |
| Fraction2 | 0.151 | −0.534 * | 0.127 | 0.440 | −0.210 | −0.311 | −0.105 | 0.219 | 0.161 | −0.459 | −0.304 | −0.045 | −0.431 | 1 | −0.203 | 0.342 | −0.272 |
| Fraction3 | 0.229 | 0.229 | −0.038 | −0.368 | 0.672 ** | −0.081 | 0.057 | −0.165 | −0.070 | 0.373 | 0.25 | 0.084 | 0.069 | −0.203 | 1 | −0.015 | −0.669 ** |
| Fraction4 | 0.084 | −0.560 * | 0.243 | 0.193 | −0.283 | −0.726 ** | 0.079 | 0.778 ** | 0.762 ** | −0.173 | −0.232 | −0.08 | −0.893 ** | 0.342 | −0.015 | 1 | −0.667 ** |
| Fraction5 | −0.369 | 0.294 | −0.009 | −0.003 | −0.195 | 0.457 | −0.115 | −0.472 | −0.484 | −0.087 | −0.018 | −0.076 | 0.483 | −0.272 | −0.669 ** | −0.667 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuso, A.; Bancalari, E.; Castellone, V.; Caligiani, A.; Gatti, M.; Bottari, B. Feeding Lactic Acid Bacteria with Different Sugars: Effect on Exopolysaccharides (EPS) Production and Their Molecular Characteristics. Foods 2023, 12, 215. https://doi.org/10.3390/foods12010215
Fuso A, Bancalari E, Castellone V, Caligiani A, Gatti M, Bottari B. Feeding Lactic Acid Bacteria with Different Sugars: Effect on Exopolysaccharides (EPS) Production and Their Molecular Characteristics. Foods. 2023; 12(1):215. https://doi.org/10.3390/foods12010215
Chicago/Turabian StyleFuso, Andrea, Elena Bancalari, Vincenzo Castellone, Augusta Caligiani, Monica Gatti, and Benedetta Bottari. 2023. "Feeding Lactic Acid Bacteria with Different Sugars: Effect on Exopolysaccharides (EPS) Production and Their Molecular Characteristics" Foods 12, no. 1: 215. https://doi.org/10.3390/foods12010215
APA StyleFuso, A., Bancalari, E., Castellone, V., Caligiani, A., Gatti, M., & Bottari, B. (2023). Feeding Lactic Acid Bacteria with Different Sugars: Effect on Exopolysaccharides (EPS) Production and Their Molecular Characteristics. Foods, 12(1), 215. https://doi.org/10.3390/foods12010215